New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 19: Flying, Space and Diving
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 19
|
 |
|
|
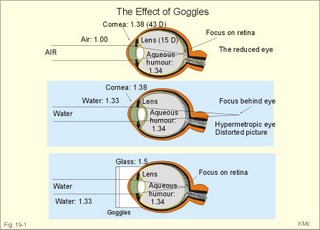
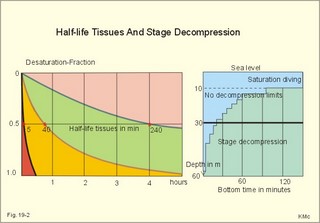
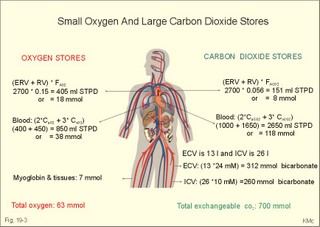
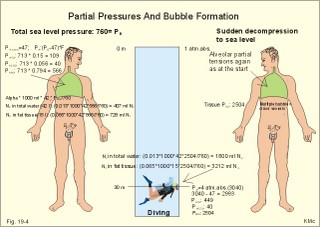
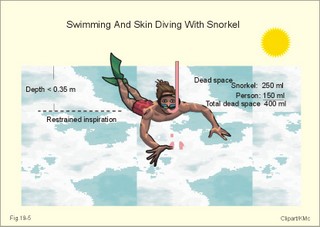
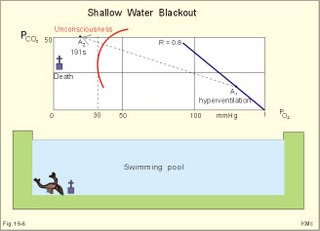
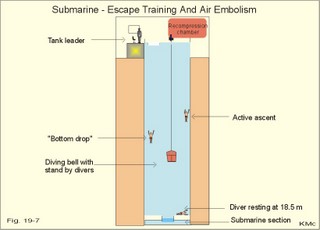
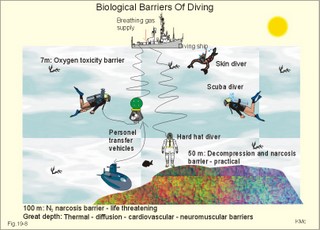
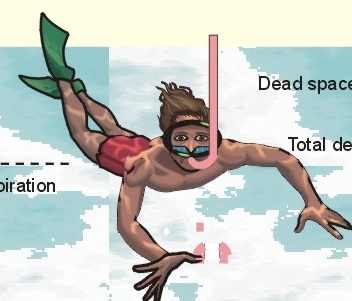
· To define the law of ideal gasses, Boyle-Mariotte´s law and Newton’s law of universal gravitation. · To describe essential topics related to flying, space life and diving, hyperbaric oxygenation and recompression therapy, inert gas narcosis and oxygen toxicity. · To calculate one variable from relevant variables given. · To explain decompression sickness, hypobarotrauma (squeeze), and alveolar rupture in the hypo- and hyperbaric environment. · To use the above concepts in problem solving and in case histories. · Newton’s law of universal gravitation: Every particle in the universe attracts every other particle with a force directly proportional to the product of the two masses and inversely proportional to the square of the distance between them. · Haldane’s law: Any saturation dive to less than 10 m does not cause decompression sickness and allows ascent without decompression stops. · Flight decompression. Fast ascent during flight rarely causes decompression problems at altitudes below 10 km (30 000 feet). · Absolute atmospheric pressure is the pressure measured in atmospheres absolute (ie, one atm.abs. at the surface of earth, and 2-3-4 atm abs at 10-20-30 m of seawater depth). · Decompression is the gradual fall in ambient pressure during ascent both in air and in water. · Decompression sickness refers to the formation of growing bubbles during decompression causing tissue ischaemia and necrosis. · Gravity units (G) refers to the gravity of the earth (G = 9.807 or approximately 10 m s-2). · Hyperbarotrauma or air embolism is a major problem of submarine escape. As the submariner escapes and ascends from the vehicle, the gas volume of the lungs expands and ruptures pulmonary vessels. Air enters the tissues, the thoracic cavity and the circulatory system. This causes air embolism and death. · Inert gas narcosis describes an intoxication syndrome with euphoria or anxiety, gross errors in performance and eventually unconsciousness. Nitrogen starts to be narcotic at depths of 40 m or more. · Pressure is measured as force per area unit. The pressure of a 10 m high sea water column resting on 1 m2 is 1 atmosphere equal to 101.3 kPa or 760 mmHg. · Recompression therapy of decompression sickness takes place in a pressure chamber, where sufficient pressure can be established to eliminate the bubbles causing the disease. · Squeeze or hypobarotrauma is caused by a negative pressure difference across the wall of a non-collapsible air space (middle ear, sinuses, compressed lungs, hollow teeth etc.). High-altitude flight, space flight, and diving encounters disorders due to change of total pressure (cf. barotrauma, decompression sickness), forces of acceleration and weightlessness, fall in oxygen pressure (oxygen deficiency), fall in ambient temperature, and increased radiation. This paragraph deals with 1. Commercial aircraft flying, 2. Satellite flying, and 3. Diving. Commercial aeroplanes fly at an altitude of approximately 33 000 ft or 10 300 m, where the ambient pressure is 26 kPa or 195 mm Hg and the oxygen tension in the outside cold air is around 40 mmHg - less than on Mt. Everest. The aeroplanes have cabins pressurised to 80 kPa (corresponding to an altitude of 2000 m or 6000 ft) or sometimes only to an altitude of 2750 m. These levels cause hypoxic dyspnoea only in cardiorespiratory patients. The main problem for the average traveller is squeeze (hypobarotrauma) of sinuses and teeth during landing. In case of depressurisation of an aircraft, PAO2 is rapidly reduced, and oxygen delivery must be sustained with an oxygen mask. Hypoxia is the acute danger; decompression sickness (see below) is avoided by fast descent to lower altitudes (higher pressures). The rhythmicity of human organ functions is coupled to the daily periodicity of the earth’s rotation. The unknown endogenous periodicities, correspond approximately (Latin: Circa) to the duration of a day (Latin: Dies), so they are called circadian rhythms. Many circadian rhythms are synchronised to the 24-hour cycle (the biological clock) by external signals such as light and darkness or social habits. The most important diurnal rhythm is the waking-sleeping cycle, with a fall in body temperature, respiratory rate and heart rate at the onset of sleep. Flight across time zones causes jet lag, which is a discrepancy between the circadian rhythms and the external signals. An eastward flight causes greater jet lag than westward flights. The adjustment of circadian rhythms takes one day for every two hours of time shift when flying east. Parachuting: The accelerator force of gravity has the same size at a given latitude. Consider a body falling freely in an environment without air molecules along a straight line perpendicular to the surface of the earth. In the absence of air resistance, all bodies fall towards the earth with the same acceleration regardless of the size and composition of the body. The magnitude of this accelleration, which is represented by the gravity unit (G), is 9.8 or approximately 10 m s-2. If a parachuter was only exposed to gravity he would fall towards the earth with the acceleration 10 m s-2. Following the first 10 s, the velocity is approximately (G* t) = (10 m s-2 *10 s) or 100 m per s. In real life there is no such thing as a free fall from an aeroplane, because the air resistance soon reduces the velocity and outbalance the acceleration. Here, the parachuter reaches the terminal velocity determined by the relation between G and air resistance. As the parachute opens, the parachuter experiences an opening shock load of 500-600 kg. The area of the standard parachute reduces the terminal velocity to 1/9. The force of impact at landing is (1/9)2 = 1/81 of the landing force without parachute. This is equal to a jump from a height of 2-3 m. Space flight requires pressure suits or cabins ensuring adequate oxygenation and pressurisation to protect against hypoxia and decompression sickness. More than 20 km out in the stratosphere (Fig. 16-9), the barometric pressure is less than the water vapour tension in alveolar air (47 mmHg or 6.3 kPa), and the oxygen tension is rapidly approaching zero. Without pressurised cabins the blood of the astronaut would boil. In the stratosphere, instantaneous decompression by pressure equalisation with the environment causes a monstrous form of decompression sickness with air emboli all over the body. The body volume of humans increases by a factor of at least three by air expansion in tissues and blood. Recycling techniques have been developed for the reuse of O2. The spacecraft - just as submarines - must carry along enough carbon dioxide absorbent to prevent death from carbon dioxide poisoning. Lethal radiation dose is rapidly reached when flying around the earth in the Van Allen radiation belts, consisting of high-energy level protons and electrons. Therefore, commercial flying take places essentially below the inner belt (500 km). Start and landing of long distance space flights take place as close to the magnetic poles - with minimum radiation energy exposure - as possible. Forces of acceleration (G) cause pooling of blood in the lower extremities, a critical drop in arterial blood pressure with orthostatic hypotension or collapse due to reduced venous return, but also cause nausea, vomiting, and spatial disorientation. The start acceleration of a spacecraft is approximately linear, and the astronaut is exposed to a tremendous acceleration - often close to 8 times the gravity of the earth (8 G) at the first stage of a 3-stage blast-off. The space shuttle implies a 3 G start and a 1.2 G landing. The body of the astronaut is located transverse to the axis of acceleration in order to prevent pooling of blood in the legs. The astronaut often carries an anti-G suit with the same purpose. Gravity acts on both the astronaut and the spacecraft in space, so astronauts are floating inside the satellite. Weightlessness, caused by the absence of gravitational forces, produces diverse reactions. The space pilot experience space sickness (nausea and vomiting over the first days), falling total blood volume, muscular atrophy, Ca2+ loss from the bones, and obstipation, brought about by the lack of stimuli. After living in outer space for weeks, adaptive difficulties develop upon return to the life on earth. These difficulties are low working capacity and a tendency to faint while standing. The bone loss continues long after the return to earth, because stimulation of bone formation requires physical activity in a gravity field. This paragraph deals with 3a. Senses in water, 3b. Inactive gasses, and 3c. Gas stores of man. The light intensity decreases rapidly with depth, and 100 m below sea level, it is permanently dark. The ratio of the speed of light in free space to its speed in a medium is called the index of refraction of the medium. The power of a lens is proportional to the change in index of refraction and inversely proportional to the radius of its curvature. Fig. 19-1: Light refraction in the reduced eye in air and in water. The effect of goggles is remarkable and shown below. The light refraction is shown in a schematised eye, where the air-cornea interface contributes 43 Diopters and the lens 15 to the focusing of parallel light rays upon the retina (Fig. 19-1). Normally, it is the difference in index of refraction between the air (1.00) and the cornea (1.38), which accounts for the major refraction at the air - cornea interface (Fig. 19-1). When water (1.33) is substituted for air, this difference becomes so small that parallel light rays are now focused behind the retina. Only maximal accommodation of the lens can refract parallel light rays but not enough to focus them on the retina. Under water the unprotected eye becomes farsighted (hypermetropic) and the image is distorted. Goggles restore the air-cornea interface. In spite of the high index of refraction of glass (1.5), the parallel light rays are not refracted, because the radius of a glass plate is infinite. Light rays not falling perpendicular to the glass are refracted, which explains why objects look larger under water. Sound propagates more rapidly in soft tissue and water than in air (1540 m s-1 as opposed to 340 m s-1 in air). In air the detectable phase- or time lag between the two ears helps us to determine the direction of the sound source. Therefore underwater sound sources appear much nearer than they actually are. Because of the shortened delay between the two ears, localisation of sound sources becomes extremely difficult. The voice of a diver speaking in a helium-oxygen atmosphere resembles the voice of Disney's Donald Duck, because the high tones dominate in this light medium. Inactive gasses are eliminated by diffusion to the peripheral blood and by convection with the blood to the lungs. During diving the ambient pressure will rise by one atmosphere for every 10 m of seawater depth you pass. Fig. 19-2: Desaturation curves showing elimination of nitrogen from tissues with different half-life. – The depth and bottom time for stage decompression is shown to the right. Inactive gasses (ie, N2, H2, He, Ne, Ar etc) contribute with aa alveolar partial pressure when present in the breathing medium, and the blood of the lung capillaries is in immediate equilibrium with the partial pressure in the alveolar air. Atmospheric air is the commonly used breathing gas for diving. The solubility coefficient for N2 in blood is low (ie, 0.012 - see Table 13-1), but five times larger in fat tissue, which has a modest perfusion rate. A large cardiac output is necessary to transport substantial amounts of N2 to the tissues. During saturation dives it takes a long tome before the nitrogen tension of fat tissue reaches equilibrium with PaN2. The human body is a complex of tissue types, each with an individual, exponential uptake rate or half-life for saturation (Fig. 19-2). Often a model with half-life tissues of 5-, 10-, 20-, 40-, 80-, 120-, 240-, 480- minutes (of half-life) is used. No decompression diving limits (cf. decompression tables) are limits of depth and time spend at the depth. A work period of one hour at 60 m of depth requires a decompression period of three hours on the steps scheduled in the decompression tables during ascent. Any saturation dive to less than 10 m allows ascent without decompression stops (Haldane's law). Fast ascent during flight rarely causes problems at altitudes below 10 km (30 000 feet). At altitudes above 20 km (where the ambient pressure is less than 6.3 kPa or 47 mmHg) the blood of a space traveller with normal body temperature will boil, if the pressure within his suit is lost. This is because the partial pressure of saturated water vapour at body temperature is 6.3 kPa or 47 mmHg. The gas stores of the human body are easy to calculate from standard values, and the calculations are shown in Fig. 19-3. The resting person has a lung volume of 2700 ml STPD (standard Expiratory Reserve Volume added to Residual Volume), and the blood volume consists of 2 l of arterial and 3 l of venous blood. The extracellular fluid volume (ECV) and intracellular fluid volume is 13 and 26 l, respectively. The oxygen content of the arterial and venous blood is 200 and 150 ml STPD per l, respectively. The bicarbonate concentration extra- and intra-cellularly is 24 and 10 mM, respectively. The carbon dioxide content of the arterial and venous blood is 500 and 550 ml STPD, respectively. The oxygen stores of the lungs are 18 mmol, and of the blood 38 mmol (Fig. 19-3). We also have small O2 stores (7 mmol) in myoglobin and in the tissues, a total of (18+38+7) = 63 mmol. Hypoxic brain damage can occur after 5 min of apnoea and after 5 s of cardiac arrest (black out and grey out). Fig. 19-3: Comparison of the small oxygen stores to the large exchangeable carbon dioxide stores of man. The CO2 stores of the lungs are 8 mmol, and those of the blood 118 mmol. The ECV of an adult person contains 312 mmol bicarbonate. Intracellularly there is 260 mmol mobile bicarbonate. These exchangeable CO2 stores comprises (8 +118+312+260) = 698 or 700 mmol in total (Fig. 19-3). The size is subject to changes by alterations of ventilation or acid-base-status. Besides, there are large amounts of CO2 fixed in bones. The human tissue stores of nitrogen (N2) are calculated in Fig. 19-4. A normal weight person contains 15 kg of fat and 42 litres of water (total water). Nitrogen is five times as soluble in lipid tissue (solubility coefficient 0.065, see Chapter 13, Table 13-1) as in water (0.013). The blood of the pulmonary capillaries is saturated with the nitrogen at the pressure prevailing in the alveolar air. Fig. 19-4: Partial pressures and bubble formation. The nitrogen content of a diver at sea level before diving (left) is compared to that depth being saturated with N2 (middle), and to the content following sudden ascent (right). At sea level before diving, the diver contains about 400 ml of N2 in the total water phase and 725 ml in the fat tissue (Fig. 19-4, left). The partial pressure of nitrogen is 566 mmHg in all tissues. When the diver reaches the bottom, there is a rise in ambient pressure to 3040 mmHg, and the new N2-gradient (2504 - 566 mmHg) will increase the uptake of N2 by the pulmonary blood. It takes hours before all fat-containing body tissues are saturated with dissolved N2 at the new pressure, because of the low bloodflow and poor capillary network of fat tissue. The total water phase is in equilibrium within an hour. The amount of nitrogen is now 1800 ml in the total water phase and about 3200 ml in the fat tissue (Fig. 19-4, middle). At depth the compression of the external pressure keeps all gasses dissolved in the body. When the diver suddenly surfaces (sudden decompression to sea level), the total pressure is suddenly only 1 atm.abs. or 760 mmHg, whereas the pressure inside the body tissues approaches that of the alveolar air, when he left the bottom (3040 out of which 2504 mmHg was nitrogen). Obviously, most of the total pressure in the tissues is due to dissolved nitrogen, and without counter-pressure the N2 -molecules are released from the dissolved state in the form of bubbles. In the case shown in Fig. 19-4, the diver is supposed to eliminate a large surplus of N2 (5000 - 1133 ml) during the rapid ascent to the surface. This is not possible for the circulation to cope with and bubbles are formed in blood and other tissues (see decompression sickness below). This paragraph deals with 1.Drowning, 2. Skin diving and disorders, 3. Disorders from hard hat and SCUBA diving, and 4. Hyperbaric oxygenation therapy. In more than 90% of drowning and near drowning the lungs are flooded with water. Fresh water is hypotonic and rapidly absorbed, diluting plasma to become hypotonic and causing bursting of the red cells (haemolysis). The victim often dies within 3 min. Seawater is hypertonic and draws fluid from the blood plasma into the lung alveoli and interstitial fluid, so that plasma volume decreases. This causes haemoconcentration and shock. The victim often dies within 6 min. The rational therapy, if early enough, is immediate resuscitation and treatment of the respiratory and circulatory disorders including haemolysis or haemoconcentration. The simplest type of diving is breath-hold diving or naked skin diving. This is performed without equipment or with only a snorkel and a mask. This is the most popular form of diving performed as underwater sports, fishing, photography, archaeology etc, and involving diving tribes as the Amas of Japan and Korea, and the Polynesian pearl divers. Breathing through a snorkel has limitations. The maximum distance between the water surface and the middle of the immersed thorax, is 0.35 m (Fig. 19-5). Fig. 19-5: Swimming and skin diving with snorkel just below the surface. This is because the water pressure creates a pressure gradient in the pulmonary vascular system, which is a low-pressure system. The intra-alveolar pressure is equal to the atmospheric pressure, so the thoracic vessels are distended with blood, and the high extra-thoracic pressure restrains the respiration. Thus, the length of the snorkel cannot be extended beyond that range. The snorkel is sometimes provided with a ball valve that closes automatically at diving. Snorkel breathing implies a dead space problem. This is because the sum of the snorkel- and person- dead space (here 400 ml) is comparable in size to the normal tidal volume of 500 ml. Thus it is necessary to increase the tidal volume. Breath-hold diving is dangerous for several reasons: 2a. Hypoxia, 2b. Cardiac arrhythmias, 2c. Air embolism, 2d. Squeeze. Prior hyperventilation with only three deep inspirations increases PAO2 to 17.3 kPa (130 mmHg), whereas PACO2 is only 2.6 kPa (20 mmHg). A well-trained swimmer can use up the oxygen stores during forceful underwater swimming, before sufficient carbon dioxide is produced to awake the desire for breathing. Hypoxia in itself is insufficient to trigger inspiration. Cerebral PO2 can fall below 4 kPa or 30 mmHg (zone of unconsciousness or grey out) without a sufficient PaCO2 is build up to force the swimmer to the surface. Drowning is likely, if this occurs underwater. This sequence of events has sometimes been called shallow water blackout. Underwater swimming record attempts should be discouraged, as they are dangerous. Unconsciousness from hypoxia and subsequent drowning has occurred among expert pool swimmers trying to set distance records in underwater swimming (Fig. 19-6). Fig. 19-6: Record attempts in apnoea underwater swimming is life threatening (+). Point I is the normal inspiratory point. Hyperventilation prior to the dive can cause acute respiratory alkalosis with dizziness and convulsions even before the dive. The initial hyperventilation to the point A1 (hyperventilation) eliminates the CO2 drive to respire, so while exercising underwater they slide into hypoxic unconsciousness (point A2 in Fig. 19-6) and death. The data for the alveolar points are obtained from a medical student, who tried to keep his breath for as long as possible (191 s) lying supine in air - and fainted. He was immediately rescued by neck extension, whereby he expired and started spontaneous breathing again - making artificial ventilation unnecessary. Deep diving with long bottom time results in anoxia during ascent. The PAO2 falls so drastically during ascent that oxygen transport is reversed in the alveoli. In cold water especially, a cutaneo-visceral reflex from cold receptors of the skin seem able to elicit malignant cardiac arrhythmia’s or a slow heart rate termed diving bradycardia or both. The latter can be beneficial to the breath-hold diver by reduction of the oxygen demand of the myocardium, but the slow heart rate may develop into a vasovagal syncope, which is lethal under water. Heart rates around 30 beats per min have been observed in small children, who have been victims of cold water near drowning for periods up to 40 min. Such heart rates are similar to those found during hypothermic surgery. These arrhythmias and bradycardias are triggered from cold receptors in the skin and from general hypothermia. The human diving response is a natural vagal reduction of heart rate occurring during breath holding on land as well as under water (diving bradycardia). Individuals with a high vagal excitability can experience sino-atrial blockage, which may develop into a severe Adam Stokes syndrome. This is fatal under water. 2c. Submarine escape training and emergency escape Escape from a submerged submarine is accomplished from more than 100 m depth without the use of equipment. The submariner ascends through the water, while exhaling continuously in a controlled manner. He is trained to follow the ascent rate of his own exhaled air bubbles. The art of the procedure is to exhale in such a way that the pulmonary pressure is only slightly elevated when surfacing. Fig. 19-7: Principal features of breath-hold diving in a submarine-escape training tank. At 90 m of depth the ambient pressure is 10 atm.abs., so with a total lung capacity of 6 l, the lungs of the submariner contain the same number of air molecules as those in 60 l of air at 1 atm.abs. according to Boyle-Mariotte´s law. If the submariner panics and try to keep all air in his lungs, the gas will expand the lungs until they rupture, and the air enters the surrounding tissues, pleural cavity and blood vessels. The air embolism can kill the submariners even before they reach the surface. When training such a controlled procedure in submarine escape training facilities (Fig. 19-7), it is important to have a recompression tank close to the surface, and means to place a victim of air embolism at high pressure within seconds. Air embolism is a hyperbarotrauma or barotrauma of ascent (Fig. 19-7). This danger is present whenever a person breathes compressed air at depth. An emergency escape even from 3 m of depth may lead to death, if the subject does not exhale. The lung volume increases during ascent according to Boyle-Mariotte´s law. The low-pressure vessels of the thoracic cavity are thin-walled. They are compressed and the alveoli dilatate until they burst, whereby the air dissects its way into the tissues (subcutaneous emphysema) and into the pleural cavity (pneumothorax). Alveolar air enters the blood through damaged vessel walls. The blood stream to the brain, heart and lungs are blocked by air (air embolism), and death ensues within minutes due to the blocked bloodflow to brain and heart. is a function of Boyle-Mariotte´s law: For a certain amount of gas the product of pressure and volume is constant at constant temperature. The hypobarotrauma is caused by a negative pressure difference across the wall of a non-collapsible air space in the body (middle ear, sinuses, hollow teeth, compressed lungs etc). A breath-hold diver who dives too deep will eventually bleed into his alveoli, and the presence of lung squeeze is evident, when he surfaces with bloodstained froth around the mouth. When a healthy person, with a total lung capacity (TLC) of 6 l and a residual volume (RV) of 1.5 l at the surface, breath-hold dive to 30 m or 4 atm.abs., his TLC is compressed to 1/4 or 1.5 l, which is equal to his RV at the surface. By calculation this depth is assumed to be the maximal diving depth. In such a calculation is implied that the RV at the surface is equal to the RV at the bottom. However, this is not true. The world record is beyond 105 m, which is unbelievable, when compared to the maximum calculated above. The following two phenomena explain this world record. RV decreases with increasing diving depth, because the diaphragm is pushed upwards as the piston in a syringe, and blood is pushed into the pulmonary circulation. At some further depth, the capillaries will rupture and blood/oedema fluid reaches the alveoli (alveolar squeeze). This is a hypobarotrauma or barotrauma of descent, and it has been observed in some of the record holders. A diver who has caught a cold, will descend with sinus openings closed, and develop pain with depth as the pressure in the occluded sinuses becomes more negative compared to the surroundings (ie, sinus squeeze). 3. Disorders from hard hat and SCUBA diving Use of a diving helmet with a hose, through which atmospheric air is pumped to the diver at depth, is the classical form of diving. The depth limit is around 50 m for air breathing, because of the danger of gas narcosis and of decompression sickness at prolonged dives (Fig. 19-8). With a hose-and-pump system the inspired air is pressurised to match the surrounding pressure, and the expired air is given off to the water. Such a device is of no use in secret operations underwater. Fig. 19-8: SCUBA and hard hat diving from ship or from personal transfer vehicles. Pathophysiological barriers of diving depth are given. Self-Contained underwater breathing apparatus (SCUBA) is an open circuit system with a demand valve at the mouth. Usually the diver receives compressed air from 2 tanks carried on his back (Fig. 19-8). First the pressure of the air stream is reduced from the initial value of 100-200 atm.abs. inside the tank, to a pressure somewhat higher than the ambient water pressure. Then the air passes an inhalation demand valve due to the negative pulmonary pressure during inspiration, and finally the expired air is exhaled into the water at a positive pressure from the expiratory muscles. Most SCUBA dives are performed to depths of 30-40 m (Fig. 19-8). SCUBA divers release bubbles in the water when breathing, so they are useless for secret tasks. The problems related to these types of diving are 3a. Decompression sickness, 3b. Inert gas narcosis, 3c. Body squeeze, 3d. Oxygen toxicity, and 3e. Carbon dioxide toxicity. If the diver ascents from the bottom to the surface too rapidly, inert gases stored in the tissue form bubbles in the blood and tissues, just as bubbles are formed when a bottle of soda water is opened. The intravascular bubbles cause tissue ischaemia and necrosis. Besides there is also serious extravascular damage. The formation of bubbles is increased by exercise, just as more bubbles are formed when the bottle of soda water is shaken up. These phenomena are gathered in the concept decompression sickness. Symptoms and signs can be present in all tissues of the body. The main problems are caused by bobbles blocking the blood supply. Pains occur in the muscles and in the joints (bends). Life-threatening bubbles may block the pulmonary capillaries, which trigger thoracic pain called chokes with alarming dyspnoea, pulmonary oedema and often death. The CNS symptoms and signs are dizziness, paralysis, collapse and unconsciousness. The ascent from deep dives must be slow and systematically in stages. Stage decompression, according to decompression tables, prevents decompression sickness in the majority of seemingly healthy persons. Stage decompression with a rate below 18 m per min between stages, allows most people to ascent without decompression sickness (bends, caisson disease). All dives no deeper than 10 m allows the diver to emerge without stage stop (Haldane’s law). The limit for compressed air diving should be 50 m (150 feet). Rational therapy requires immediate recompression in a pressure chamber, where sufficient pressure can be established to eliminate the bubbles causing the disease. The nitrogen gradient from the divers body to the air in the decompression tank is preferably increased (with oxygen enriched air) for rapid removal of nitrogen from the body. Recompression can be succesful even hours after the dive. Diving at great depth (80-300 m) makes it necessary to live in large habitats at depth for longer periods. These divers are saturated with the inert gas (He, Ar etc) to which they are exposed, and this type of diving is termed saturation diving. Helium is used together with a small O2 fraction, in order to avoid acute O2 poisoning (see below). At 200 m of depth only 1% O2 is necessary in the helium-oxygen mixture (so-called heli-ox-mixture). The oxygen pressure in the inspired air is (21 atm.abs. * 0.01) = 0.21 atm.abs. or 160 mmHg. Dives deeper than 50 m are performed with helium instead of nitrogen as the inert gas. The advantages of helium is a low solubility coefficient in the tissues, a low narcotic-toxic effect, a rapid diffusion rate out of the tissues, and a minimal airway resistance due to its low density. The breathing resistance due to the high density of nitrogen at great depth makes it almost impossible to perform manual work with nitrogen as the inert gas. Cases of decompression sickness have occurred as a result of breath-hold diving in submarine escape training tanks and among the skin-diving pearl divers of the Tuamotu Archipelago in the South Pacific. When severe it comprises loss of consciousness and paralysis of one or more limbs, and is often fatal. Repetitive skin diving to great depths for prolonged periods in warm and ideal waters must be avoided. Rapture of the depth - or nitrogen narcosis - appears at 40 m when breathing compressed air. The diver becomes euphoric with behaviour similar to alcoholic intoxication (ie, lack of judgement and concentration, incoordination, anxiety). The inert gas narcosis increases in intensity with depth according to Martini’s law: Each 10 m of diving depth changes the behaviour as much as one drink. Therefore, the limit for compressed air diving should be 50 m (Fig. 19-8). At a depth of 90 m or more a typical narcotic condition develops (anaesthesia and unconsciousness). Inert (inactive) gases are lipid-soluble and dissolve easily in fatty tissues, cell membranes and intracellular structures, where they bind to active sites or receptors. The gases modify neuronal activity and nerve conduction velocity as well as ionic transport across cell membranes. Argon has a larger narcotic effect than nitrogen (larger lipid-solubility, larger energy content or van der Waal-forces), and nitrogen is a much stronger anaesthetic than helium. was a dramatic event of the hard hat diving period. Accidental disrupture of the air hose to the diver working at depth suddenly exposed the diver to an internal pressure of 1 atm.abs. while the ambient water pressure could be 6 atm.abs or more (Fig. 19-8). Before the diver was rescued the soft tissues of his body was virtually compressed into the helmet. Such accidents lead to the development of contra-valves in the helmet. Active oxygen or oxygen free radicals (such as the superoxide O2- and hydrogen peroxide) are continuously produced in the mitochondria from the dissolved oxygen. As long as the oxygen tension of the tissues is normal, the production equals the removal by tissue enzymes. These enzymes can be inactivated following breathing of 100% oxygen above 2 atm.abs for longer periods. Therefore oxygen free radicals accumulate to a degree that is lethal for cells in particular brain neurons. The acute cerebral oxygen intoxication is presented with fasciculation of the mimic face muscles, vertigo, universal cramps and coma. The retinal cells of the eye are actually brain cells. Acute oxygen intoxication in premature babies may cause retinal vasoconstriction, vessel wall proliferation and retinolysis - so-called retrolental fibroplasia. Closed or recirculation systems with pure oxygen are used by frogmen for secret tasks underwater. The acute oxygen toxicity limits the O2 diver to dives not deeper than 7 m (Fig. 19-8), and duration less than 75 min. This depth is equal to a total pressure of (1.7 * 760) or 1292 mmHg (= 172 kPa). The PIO2 of the saturated tracheal air is (1292 - 47)= 1245 mmHg, implying a PAO2 which is toxic to the CNS. Recirculation systems with pure oxygen are so hazardous that they should be prohibited for sport divers. Chronic oxygen toxicity frequently develops when a patient has been breathing 80% oxygen or more at one atm.abs. for more than 12 h. Firstly, the patient develops pulmonary hypertension with typical retrosternal pain, dyspnoea, coughing, and pulmonary oedema with bloodstained frothy sputum. Secondly, the patient develops atelectasis, because the surfactant is inactivated by the toxic free radicals. The exposure of lung tissue to high oxygen tension is direct and total without the protection of the haemoglobin or any other oxygen buffer system. Some patients actually die of hypoxia in spite of the high oxygen tension! Equipment with a large dead space permits accumulation of carbon dioxide with rebreathing. This is a problem with hard hat diving and SCUBA diving with large facemasks (Fig. 19-8). The highest permissible FICO2 is 0.005 or 0.5%. Slightly higher concentrations are harmful. Firstly, the diver increases ventilation, but soon after exposure to 5% or more, the respiratory centre of the medulla is suppressed and ventilation becomes insufficient. An acute respiratory acidosis develops, and finally carbon dioxide narcosis (anaesthesia and unconsciousness) is present. 4. Hyperbaric oxygenation therapy Hyperbaric oxygenation therapy is used for disorders with either local or global oxygen deficiency with oxygen at approximately 2 atm.abs. These conditions are accomplished in pressure tanks with atmospheric air, such as those used for recompression treatment of decompression sickness. Oxygen is administered through a mask at ambient pressure. Acute O2 poisoning is the risk. Anaerobic bacilli cause Clostridial infections. Clostridium botulinum causing botulism, and clostridium tetani causing tetanus, both produce neurotoxins, whereas clostridium perfringens and septicum - causing gas gangrene - produce enzymes. The clostridia bacilli are spore forming and survive for years in our surroundings. The bacilli require anaerobic conditions to grow. See infectious disorders in Chapter 33. The botulism- neurotoxins cause cholinergic and neuromuscular blockade leading to strabismus and respiratory insufficiency (mortality rate: 70%). - The tetanus-neurotoxins are tetanospasmin causes muscle spasms ending in neuromuscular blockade and death. - Gas gangrene in lacerated wounds is a life-threatening condition. The treatment of these three Clostridial disorders with antitoxins is sometimes supported by hyperbaric oxygenation. The oxygen free radicals are supposed to inhibit the growth of the clostridia. The therapeutic results - often some benefit in moribund patients - are controversial. Air embolism, decompression sickness, CO-poisoning and leprosy have been treated with some benefit with hyperbaric oxygen. (See Chapter 13: Eq. 13-3 to 13-6) I. Each of the following five statements have True/False options: A: The normal length of a snorkel is 0.6 m. B: A PAO2 below 30 mmHg is the usual threshold for unconsciousness. C: Air embolism is always easy to separate from decompression sickness. D: The botulism- neurotoxins cause adrenergic and neuromuscular blockade. E: The acute oxygen toxicity limits the O2 diver to dives not deeper than 7 m. II. Each of the following five statements have True/False options: A. The cornea-air interface becomes a cornea-water interface, when in water without goggles. The refractive index for water and for cornea itself is much the same, eliminating most of the refractive power in air. A skin diver without goggles is hypermetropic under water. B. Sound propagates more rapidly in soft tissue and water than in air (440 m s-1 as opposed to 340 m s-1). Therefore underwater sound sources appear much nearer than they actually are. C. The solubility coefficient for nitrogen in fat is 10 times larger than in blood. D. Slightly higher concentrations of carbon dioxide than 0.5% in the inspired air are harmful to humans. E. Lethal radiation dose is rapidly reached, when flying around the earth in the Van Allen radiation belts, consisting of high-energy level protons and electrons. Therefore, commercial flying takes place essentially below the inner belt (500 km). A male farmer, 18 years of age, is stabbed through the left foot while working as a stableman. He develops a fulminate infection with clostridium tetani, which only grows under anaerobic conditions and produce a potent toxin causing permanent depolarisation of the motor end plate. He is brought to hospital in a moribund condition, which is not improved by antitoxin and antibiotics. The farmer is transferred to a pressure chamber, where hyperbaric oxygen therapy is provided at 3-atmosphere pressure for 20 min. The haemoglobin concentration of the patient is 170 g per l. PB is one atmosphere (760 mmHg), PACO2 is 5.3 kPa (40 mmHg), and the alveolar water vapour tension (Pwater ) is 6.5 kPa (47 mmHg). The Bunsen solubility coefficient (a) for oxygen in the blood is 0.022 ml STPD per ml and per mmHg. One mmHg equals 133.3 Pascal. 1. Calculate the concentration of physically dissolved O2 in the blood leaving the lung capillaries of the patient. 2. Calculate the concentration of chemically bound O2 in the blood. 3. Does excessive oxygenation lead to gas transport problems? A tall, lean diver, with a body weight of 80 kg, is working 30 m below the surface of the sea in a standard divers suit for one hour. The relative density of seawater is 1033 kg m-3. The diver is breathing atmospheric air at the environmental pressure, delivered by a pump system to his helmet. The ventilation is effective, and the diver has normal alveolar gas tensions (PaO2 is 100 mmHg and PaCO2 40 mmHg). The pressure at the surface of the sea is 1 atmospheres absolute (atm abs) or 101.3 kPa or 760 mmHg and the water vapour pressure of body warm alveolar air is 47 mmHg or 6.25 kPa. 1. Calculate the total pressure in kPa and in atm abs at 30 m of depth. 2. Calculate the partial pressure of nitrogen in the alveolar air of the diver at the surface and at the bottom. 3. Is it advisable to drag the diver to the surface without step decompression? Try to solve the problems before looking up the answers. · Fast ascent during flight rarely cause problems at altitudes below 10 km (30 000 feet). · The aeroplanes have cabins pressurised to 80 kPa (ie an altitude of 2000 m or 6000 ft) or sometimes only to an altitude of 2750 m. These levels cause hypoxic dyspnoea only in cardiorespiratory patients. · The main problem for the average traveller is jet lag, squeeze (hypobarotrauma) during take-off or Hyperbarotrauma during landing. · The area of the parachute reduces the terminal velocity to 1/9. The force of impact at landing is (1/9)2 = 1/81 of the landing force without parachute. This is equal to a jump from a height of 2-3 m. · The space pilot experience space sickness (nausea and vomiting), falling total blood volume, muscular atrophy, Ca2+ loss from the bones, and after living in outer space for weeks, adaptive difficulties to life on earth upon return. These difficulties are low working capacity and a tendency to faint. The bone loss continues long after the return to earth, because stimulation of bone formation requires physical activity in a gravity field. · At altitudes above 20 km, where the ambient pressure is less than 47 mmHg or 6.5 kPa, the blood of the space traveller will boil, if he is suddenly exposed because the pressure within his suit is lost. · Any saturation dive to less than 10 m allows ascent without decompression stops (Haldane’s law). · If decompression is too rapid, inert gasses stored in the tissues form bubbles in the blood and tissues (decompression sickness). · Drowning. Fresh water drowning kills the victim rapidly following haemolysis. Seawater is hypertonic and draws blood from plasma to the alveoli causing haemoconcentration and shock. · Shallow water blackout is a condition where the oxygen stores are used up during forceful underwater swimming before sufficient carbon dioxide is produced. · Carbon dioxide poisoning results in acute respiratory acidosis and finally in carbon dioxide narcosis. · Oxygen poisoning is caused by active oxygen or oxygen free radicals (such as the superoxide O2- and hydrogen peroxide). · Rapture of the depth - or nitrogen narcosis - appears at 40 m when breathing compressed air. The diver becomes euphoric with behaviour similar to alcoholic intoxication (ie, lack of judgement and concentration, incoordination, anxiety). · Hypobarotrauma (squeeze) is a barotrauma of descent and a function of Boyle-Mariottes law. · Hyperbarotrauma (air embolism) is a barotrauma of ascent during emergency escape. The danger is present whenever a person breathes compressed air at depth. · Clostridial infections are treated with antitoxins and sometimes supplied with hyperbaric oxygenation. Accumulation of oxygen free radicals kills the microorganisms. Some cases of air embolism, decompression sickness, CO-poisoning and leprosy have been treated. Bennett, PB, and Elliott, DH (2004) The Physiology and Medicine of Diving. 5th Ed. Philadelphia: WB Saunders Co. Paulev, P-E, Pokorski, M, Honda, Y, Ahn, B, Masuda, A, Kobayashi, T, Nishibayashi, Y, Sakakibara, Y, Tanaka, M, and Nakamura, W. Facial Cold Receptors and the Survival Reflex "Diving Bradycardia" in Man. Jpn. J. Physiol.40: 701-712, 1990. Undersea & Hyperbaric Medicine. Journal published by the Underwater and Hyperbaric Medical Society, 10531 Metropolitan Av., Kensington MD 20895, USA. West JB. Respiratory Physiology – the essentials. 8th Ed. Williams & Wilkins, Baltimore, USA, 2008.
|
||
Click here to introduce your comments or contributions