New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 24 : Body Fluids and Regulation
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 24
|
 |
|
|
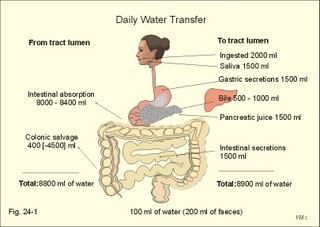
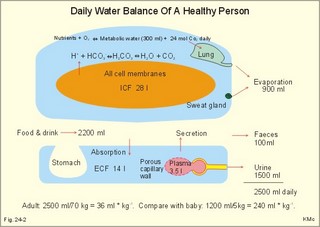
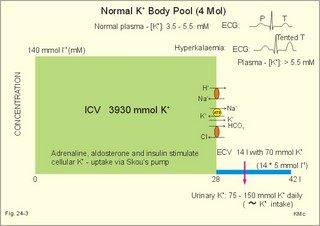
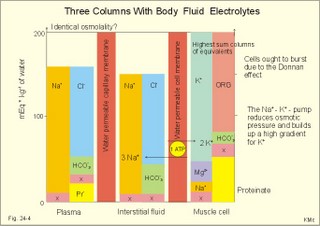
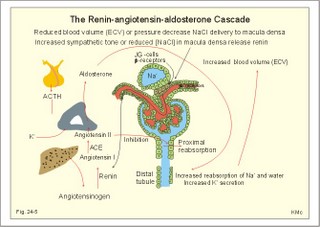
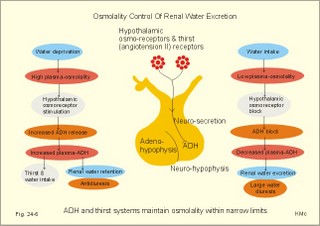
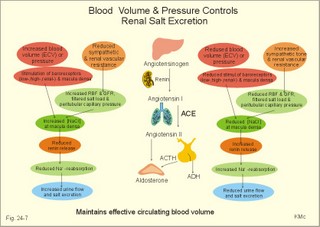
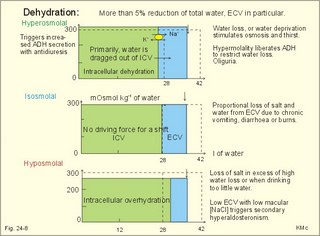
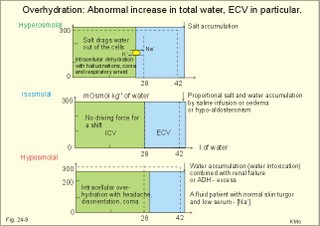
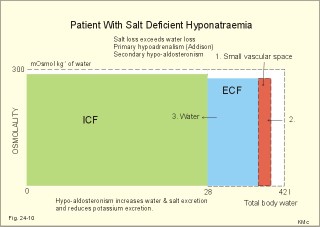
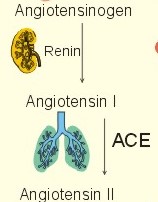
· To define the concepts: Dehydration, hyponatraemia, intracellular fluid volume (ICV), extracellular fluid volume (ECV), interstitial fluid (ISF), overhydration, oxidation water, radioactivity, specific activity, and total body water. · To describe the daily water balance, the K+- and Na+-balance, sweat secretion, the ionic composition in blood plasma, the water content of fat- and muscle- tissue and the daily water transfer across the gastro-intestinal mucosa. To describe the osmotic pressure in the body fluids, the measurement of fluid compartments by indicator dilution, the measurement of total body-K+ and -Na+ and the related dynamic pools. · To draw models of the body fluid compartments. · To explain the influence of age, sex and weight on the size of the total body water and its phases. To explain disorders with increased or reduced extracellular fluid volume and shock. · To apply and use the above concepts in problem solving and in case histories. · The law of conservation of matter states that mass or energy can neither be created nor destroyed (the principle of mass balance). The principle is here used to measure physiological fluid compartments and the body content of ions. · Concentration: The concentration of a solute is the amount of solute in a given fluid volume. · Dehydration is a clinical condition with an abnormal reduction of one or more of the major fluid compartments (ie, total body water with shrinkage of blood volume or ISF). · Dextrans are polysaccharides of high molecular weight. · Intracellular fluid volume (ICV) refers to the volume of fluid inside all cells. This volume normally contains 26-28 litre (l) out of the total 42 l of water in a 70-kg person. - One litre of water equals one kg of water. · Extracellular fluid volume (ECV) refers to the interstitial and the plasma volume. The ECV contains the remaining water (14-16 kg) with most of the water in tissue fluid (ISF) and about 3 kg of water in plasma. - Interstitial fluid (ISF) is the tissue fluid between the cells in the extravascular space. · Hyperkalaemia refers to a clinical condition with plasma-[K+] above 5 mM (mmol/l of plasma). · Hypokalaemia refers to a clinical condition with plasma-[K+] below 3.5 mM. · Hypernatraemia refers to a clinical condition with plasma-[Na+] above 145 mM. · Hyponatraemia refers to a clinical condition with plasma-[Na+] below 135 mM. · Oedema refers to a clinical condition with an abnormal accumulation of tissue fluid or interstitial fluid. · Osmolality is a measure of the osmotic active particles in one kg of water. Plasma-osmolality is given in Osmol per kg of water. Water occupies 93-94% of plasma in healthy persons. Plasma osmolality is normally maintained constant by the antidiuretic hormone feedback system. · Overhydration refers to a clinical condition with an abnormal increase in total body water resulting in an increased ECV and thus salt accumulation. · Oxidation water or metabolic water (oxidative phosphorylation) refers to the daily water production by combustion of food - normally 300-400 g of water daily in an adult. · Radioactivity is measured as the number of radioactive disintegrations per s (in Becquerel or Bq per l). One disintegration per s equals one Bq. · Total body water is destributed between two compartments separated by the cell membrane: The intracellular and the extracellular fluid. This paragraph deals with 1. The three major fluid compartments, 2. Water balance, 3. Body potassium, 4. Body sodium, 5. The indicator dilution principle, 6. The renin-angiotensin-aldosterone cascade, 7. Output contol, 8. Regulation of renal water excretion, and 9. Regulation of renal sodium excretion. Read first about the nephron (paragraph 1 of Chapter 25). 1. The three major fluid compartments The three major body fluid compartments are the intracellular fluid volume (ICV), the interstitial fluid volume (ISV) and the vascular space (Chapter 1, Fig.1-4). Water permeable membranes separate the three compartments, so that they contain almost the same number of osmotically active particles per kg. The three compartments have the same concentration expressed as mOsmol per kg of water or the same freeze-point depression. They are said to be isosmolal, because they have the same osmolality. The so-called lean body mass, which means a body stripped of fat, contains 0.69 parts of water (69%) of the total body weight in all persons. - Such high values are observed in the newborn and in extremely fit athletes with minimal body fat. Babies have a tenfold higher water turnover per kg of body weight than adults do. As an average females have a low body water percentage compared to males. Such differences show sex dependency, but the important factor is the relative content of body fat, since fat tissue contains significantly less water (only 10%) than muscle and other tissues (70%). This is why the relative water content depends upon the relative fat content. The average for most healthy persons is 60% of the body weight. Sedentary, overweight persons contain only 50-55 % water dependent on the body fat content. The relative content of body fat rises with increasing age and body weight, and the relative mass of muscle tissue becomes less. Consequently, the body water fraction falls with increasing body weight and age. Aging implies loss of cells, but the ECV is remarkably constant through life and under disease conditions. Each body (weight 70 kg) contains 4 mol of both sodium and potassium (ie, the total ion pool). A minor fraction of the potassium is radioactive. The calcium and magnesium content is 25 and 1 mol, respectively. In the renal tubule cells the epithelium is a single layer of cells, joined by junctional complexes near their luminal border (Fig. 25-7). Solutes can traverse the epithelium through transcellular or paracellular pathways. Virtually every cell membrane in the body contains the Na+- K+-pump, which maintains the low intracellular Na+-concentration and develops the negative, intracellular voltage. In the renal tubule cells the Na+- K+-pump, is located in the basolateral membrane. Read more about the nephron in Chapter 25 and about hormonal control later in paragraph 8 and 11 of this Chapter. Unfortunately, the simple laws of dilute solutions are unprecise at physiological concentrations. Rough estimates are based on the assumptions that extracellular sodium is associated with monovalent anions and that deviations in osmolality are twice the deviation in plasma sodium concentration. ICV: The dominating intracellular solute is potassium (K+), balanced by phosphate and anionic protein, whilst the dominating extracellular solute is NaCl. All compartments have almost the same osmolality 300 mOsmol* kg-1 of water. The thin cell membrane - or the endothelial barrier between ISF and plasma in the vascular phase - cannot carry any important hydrostatic gradient. Water passes freely between the extra- and intra-cellular compartment, as osmotic forces govern its distribution and the membranes are water permeable.Fig. 24-1: The daily water transfer across the gastrointestinal barrier in a healthy standard person. The ICV comprises 26-28 kg out of the total 42-kg of water in a 70‑kg person (Fig. 1-4). ECV: The ECV compartment comprises the remaining water (14-16 kg) with most of the water in tissue fluid (interstitial fluid or ISF) and 3 kg of water in plasma (Chapter 1, Fig. 1-4). The size of the ECV compartment is proportional to the total body Na+. Changes in plasma osmolality indicate problems in water balance. A [Na+] in ECV of 150 mmol per kg of plasma water corresponds to a total osmolality of 300 mOsmol per kg. Alterations in plasma-[Na+] (osmolality) will be followed by similar changes of the ECV osmolality, because the permeability of of the capillary barrier for Na+ and water is almost equal. The daily water transfer across the gastrointestinal tract amounts to approximately 9 l in each direction (Fig. 24-1). A healthy person on a mixed diet in a temperate climate receives 1000 ml with the food and drinks 1200 ml daily. Balance is maintained as long as the water loss is the same (Fig. 24-2). Fig. 24-2: The daily water balance in a 70-kg healthy person on a mixed diet. The apparent imbalance between input (2200 ml) and output (2500 ml) is covered by 300 ml of metabolic water. Water is lost in the urine (1500 ml), in the stools (100 ml), in sweat and evaporation from the respiratory tract (900 ml) as a typical example. The total loss of water is 2500 ml, and this corresponds perfectly to the intake plus a normal production of 300 ml of metabolic water per 24 hours (Fig. 24-2). The daily dietary intake of potassium varies with the amount of fruit and vegetables consumed (75-150 mmol K+daily). More than 90% of the body potassium is located intracellularly. Only a few percent of the K+ in the body pool are found outside the cells and subject to control (Fig. 24-3). The main renal K+-reabsorption is passive and paracellular through tight junctions of the proximal tubules. Moreover K+-excretion can vary over a wide range from almost complete reabsorption of filtered K+ to urinary excretion rates in excess of filtered load (ie, net secretion of K+). The Na+-K+-pump located in the cell membrane, maintains the high intracellular [K+] and the low intracellular [Na+]. The energy of the terminal phosphate bond of ATP is used to actively extrude Na+ and pump K+ into the cell. The membrane also contains many K+ - and Cl- -channels, through which the two ions leak out of the cell. In myocardial cells, as in skeletal muscle and nerve cells, K+ plays a major role in determining the resting membrane potential (RMP), and K+ is important for optimal operation of enzymatic processes. Under normal conditions, the RMP of the myocardial cell is determined by the dynamic balance between the membrane conductance to K+ and to Na+. As [K+]out is reduced during hypokalaemia, the membrane depolarises causing voltage-dependent inactivation of K+-channels and activation of Na+-channels, allowing Na+ to make a proportionally larger contribution to the RMP.Fig. 24-3: The total body K+-pool in a healthy person comprises 4000 mmol with more than 90% intracellularly. The normal ECG and the ECG of a patient with hyperkalaemia is shown to the right. The K+ -permeability is around 50 times larger than the Na+ -permeability, so the RMP of normal myocardial cells (typically: -90 mV) almost equals the equilibrium potential for K+ (-94 mV). The excretion of K+ by overload is almost entirely determined by the extent of distal tubular secretion in the principal cells. Any rise in serum [K+] immediately results in a marked rise in K+-secretion. This transport mechanism is controlled by aldosterone and by K+. Aldosterone stimulates the secretion of K+ and H+ by the principal cells of the renal distal tubules and collecting ducts (Fig. 25-11). This is why chronic acidosis decreases and chronic alkalosis increases K+-secretion. – Actually, acute acidosis may reduce K+-secretion. Of the consumed K+, 75-150 mmol is daily absorbed in the intestine. Since 90% is excreted renally in a healthy person, there must be a minimum in a typical volume of 1500 ml of daily urine with a concentration of (75/1.5) = 50 mM. Normal urinary [K+] is at least 30 mM. A high urinary [K+] is indicative of a high total body K+ or a high intake of K+. The normal excretion fraction (Chapter 25) for K+ is 0.10 (10% or 90 mmol of the 900 mmol in the daily filtrate) corresponding to the daily intake (Fig. 24-4). A K+ -poor diet leads to hypokalaemia with less than 20 mmol K+ in the daily urine. A K+ -rich diet triggers a large secretion and a high excretion in the urine (Box 25-1). A low urinary [K+] is indicative of a low total body K+ or of extracellular acidosis with transfer of K+ from the cells in exchange of H+. A low [K+] in the distal tubule cells reduces the K+ -excretion.The normal plasma-[K+] level is dependent upon the exchange with the cells, the renal excretion rate, and the extrarenal losses through the gastrointestinal tract or through sweat.Measurement of total and exchangeable body potassium Our natural body potassium is 39K, but we also contain traces of naturally occurring radioactivity (0.00012 or 0.012% is 40K with a half-life of 1.3×109 years). When using this natural tracer, injection of radioactive tracer is avoided. The person to be examined is placed in a sensitive whole body counter, and the total activity of the tracer 40K in the body (S Bq) is measured. Specific activity (SA) is the concentration of radioactive tracer in a fluid volume divided by the concentration of naturally occurring, non-radioactive mother-substance. The concentration of mother-substance is traditionally measured in mmol per l (mM). SA is equal to radioactivity (A) per non-radioactive mass unit, m (ie, A/m in Bq/mol). Following even distribution, the SA for a certain substance must be the same all over the body. SA is preferably measured in plasma (with scintillation counters or similar equipment). Specific activity (SA) is here the number of Bq 40K per mol of mother substance (39K) in the whole body. We can calculate all 39K or total body potassium: S/SA mol per whole body - when SA is known to be 0.012% or a fraction of 0.00012. The total body potassium of a healthy person is 4000 mmol. The SA of 40K implies a 40K/39K ratio of 0.48 mmol/4000 mmol (=0.00012). An exchangeable ion pool in our body is the dynamic part of the total specific ion content. The remaining content is fixed as insoluble salts in the bones. The dynamic character implies the use of a dilution principle to measure such a pool. In order to measure the exchangeable body potassium pool, a radioactive tracer is injected, such as 42K with a physical half-life of 12 hours (12.4 hours) and urine is collected. The first urine sample is from the first 12 hours, and the second sample is covering 12 - 24 hours. The total tracer dose given must be adjusted for by the loss of tracer in the urine and by the radioactive decay during the first 12 hours mixing period. The two urine samples obtained are examined for tracer and for natural potassium. The tracer is assumed to distribute just as natural potassium after 12 - 24 hours. When the tracer is distributed evenly in the exchangeable body potassium, its SA must be the same in urine, plasma or elsewhere in the body. The exchangeable body potassium is calculated by Eq. 24-2 . The specific activity for the tracer (SA Bq per mol) is known from the plasma measurements. In this way we measure the exchangeable body potassium. The normal values are 41 mmol 39K per kg body weight for females, and 46 mmol per kg for males. The exchangeable body sodium is easy to measure using the dilution principle and a minimum of equipment. Our natural non-radioactive body sodium is 23Na. We administer the radioactive tracer, 24Na, with a physical half-life of 15 hours. We have to use a total period of 30 hours to secure even distribution in the ECV. The total tracer dose given, must be adjusted for by the loss of tracer in the urine, and the radioactive decay of 24Na (see the decay law in Chapter 1). The exchangeable body sodium is calculated by Eq. 24-2. We know the specific activity for the tracer (SA Bq/mol) from the plasma measurements; therefore calculation of the exchangeable body 23Na is easy. The normal value for exchangeable body sodium is 40 mmol/kg of body weight. In a patient with a body weight of 75 kg the exchangeable sodium is (75 × 40) = 3000 mmol. The non-exchangeable sodium is fixed in the bones. The total body sodium is measured following discrete radiation with a method called neutron activation analysis. The whole body of the patient is exposed to radiation with neutrons. A small fraction of the natural 23Na now becomes radioactive sodium (24Na) by uptake of an extra neutron. A sensitive whole body counter records the radiation from 24Na. Now we can calculate the total body sodium. Normally, the total body sodium is 1000 mmol larger than the exchangeable sodium due to the fixed sodium content of the bones (1000 + 3000 mmol = 4000 mmol 23Na). Fig. 24-4: Body fluid electrolytes. Water permeable membranes separate the three compartments, which contain almost the same number of osmotically active particles per kg. The sum columns of electrolyte equivalents in muscle cells are essentially higher than the extracellular sum columns of equivalents, because cells contain proteins, Ca2+, Mg2+ and other molecules with several charges per particle (Fig. 24-4). The above columns show the ionic composition per kg of water, so we have 150 mmol of Na per kg of plasma water. Normally, one litre of plasma has a weight of 1.040 kg and contains 10% of dry material. Consequently, one litre of plasma contains 0.940 l of water, and the rest consists of plasma proteins and small ions. Thus the fraction of water in plasma (Fwater) is typically 0.94. 5. The indicator dilution principle Mass conservation is always the underlying principle. The amount of indicator n mol distributes in V litres of distribution volume. We measure the concentration Cp in mM, following even distribution, and calculate V: V = n/Cp. Errors: Uneven distribution of indicator introduces a systematic error. - A non-representative concentration of indicator in the plasma makes it insufficient to correct for plasma proteins alone. - Loss of indicator to other compartments is inevitable. - Elimination or synthesis of indicator in the body occurs as frequent errors. - The indicator may be toxic or in other ways change the size of the compartment to be measured. Total body water, ECV, plasma volume, and the elimination rate constant are measured as follows: Total water is measured by the help of the dilution principle. Tritium marked water is a good tracer. The equilibrium period is 3-6 hours. n mol of indicator divided by Cp mmol of indicator per l is equal to the distribution volume (V) for the indicator. Healthy adolescents and children have normal values around 60% of the body weight assuming one l of water to be equal to one kg. Adult males and females with a sedentary life style and larger fat fractions contain only 50% of water.5 b. The extracellular fluid volume (ECV) is measured by administration of a priming dose of inulin intravenously. Then inulin is infused to maintain a steady state with constancy of the plasma concentration of inulin (Cp). The patient then urinates, and the infusion is stopped with collection of a plasma sample. For the next 10 hours the patient collects his urine, which makes it possible to measure all the body inulin present at the end of the infusion (n mol) assuming all inulin excreted. Dividing n with Cp gives the volume of distribution (V) after correcting for the difference in protein concentration between plasma and ISF (Eq. 24-1). Chromium-ethylene-diamine-tetra-acetate (51Cr-EDTA) is a chelate with a structure that cannot enter into cells. The chelate molecule contains radioactive Cr, making it easy to measure. The 51Cr-EDTA distributes and eliminates itself in the extracellular fluid volume (ECV) just as inulin and is therefore used to measure ECV. – For clearance measurements, we inject a single dose intravenously, and draw blood samples every hour for 5 hours. The clearance of 51Cr-EDTA is independent of Cp and a good estimate of GFR just like the inulin clearance. Since the indicator is cleared from the ECV only, it is possible to measure its size. Such methods - including renal lithium reabsorption - are important during renal function studies. Normal values for ECV are approximately 20% of the body weight or 14-17 kg. Chronically ill patients with debilitating diseases often maintain their ECV remarkably well in spite of marked reductions in the cell mass of their body.Also here, the dilution principle is used. The indicator for plasma volume can be Evans Blue (T1824) that binds to circulating plasma albumin. A small dose of albumin, marked with radioactive iodine, is also a good indicator (iodine 131 has a physical half-life of 8 days). The indicator concentration in plasma (Cp) is measured every 10-min for an hour after the administration, and the log of Cp is plotted with time. Extrapolation to the time zero determines the maximum concentration of indicator in plasma. This corrects for the biological loss, while the indicator distributes itself in the plasma phase. The tracer dose divided by Cp at time zero provides us with the intravascular plasma volume. Normal values for the plasma volume are close to 5% of the body weight. In diabetics and hypertensive patients the tracer is lost more readily through their leaky capillaries to the interstitial fluid than in healthy persons (increased transcapillary escape). 6. The renin-angiotensin-aldosterone cascade Macula densa is described in paragraph 9 of Chapter 25.The most likely intrarenal trigger of the renin-angiotensin-aldosterone cascade is the falling NaCl concentration of the reduced fluid flow at the macula densa in the distal renal tubules (Fig. 24-5). The NaCl concentration at the macula densa falls, when we lose extracellular fluid, move into the upright position and when the blood pressure falls. Renin is a proteinase that separates the decapeptide, angiotensin I, from the liver globulin, angiotensinogen. When angiotensin I passes the lungs or the kidneys, a dipeptide is separated from the decapeptide by angiotensin converting enzyme (ACE). This process produces the octapeptide, angiotensin II. Angiotensin II has multiple actions that minimize renal fluid and sodium losses and maintain arterial blood pressure. 1. Angiotensin II stimulates the aldosterone secretion by the adrenal cortex, and through this hormone it stimulates Na+-reabsorption and K+-(H+)-secretion in the distal tubules (Fig. 24-5). - Angiotensin II is in itself a potent stimulator of tubular Na+-reabsorption. 2. Angiotensin II inhibits further renin release by negative feedback. 3. Angiotensin II constricts arterioles all over the body including a strong constriction of the efferent and to some extent also the afferent arteriole. Hereby, the renal bloodflow (RBF) and to a lesser extent the glomerular filtration rate (GFR) is reduced. 4. Angiotensin II inhibits the absolute proximal tubular reabsorption – contributing to the reduction of GFR. 5. Angiotensin II enhances sympathetic nervous activity. Fig. 24-5: The renin-angiotensin-aldosterone cascade. Sympathetic stimulation of the renal nerves stimulates renin secretion directly via b-adrenergic receptors on the JG cells just as falling blood pressure in the preglomerular arterioles. - b-blocking drugs and angiotensin II inhibit the renin secretion (Fig 24-5). The combined effects from the whole renin cascade is extracellular fluid homeostasis. In contrast, exposure to stress and painful stimuli triggers the combined sympatho-adrenergic system with release of catecholamines, gluco- and mineralo-corticoids, and ACTH from the hypophysis. ACTH stimulates further the secretion of the glucocorticoid, cortisol, from the adrenal cortex. The body uses output control, when it is overloaded with water or with sodium.The most important osmotically active solute in ECV is NaCl, because it only passes into cells in small amounts. Urea, glucose and other molecules with modest concentration gradients are without importance, because they distribute almost evenly in the fluid compartments. Healthy persons use two primary control systems: 1) The osmolality (osmol per kg of water) or ion concentration controls our elimination of water. 2) The change of blood volume (ECV) or pressure controls sodium excretion - not osmolality.Only when the arterial blood pressure falls drastically the body will drop its protection of normal concentration. In such a disease state large amounts of ADH molecules are released in an attempt to improve the volume and blood pressure.8. Regulation of renal water excretion The primary control of the renal water excretion is osmolality control (Fig. 24-6). Since 2/3 of the body water normally is located within the cells, this is also an intracellular volume control.Following water deprivation even an increase in plasma osmolality of only one per cent stimulates both the hypothalamic osmoreceptors and similar (angiotensin-II-sensitive) thirst receptors. Thirst may increase the water intake of the individual and thus increase the ECV, with negative feedback to the thirst receptors. Activation of the hypothalamic osmoreceptors and thirst receptors increases the hypothalamic neurosecretion to the neurohypophysis and releases antidiuretic hormone (ADH or vasopressin). Hyperosmolality elicits a linear increase in plasma ADH, which causes water retention (Fig. 24-6) until isosmolality is reached.ADH increases the reabsorption of water from the fluid in the renal cortical and medullary collecting ducts. ADH binds to receptors on the basolateral surface of the tubule cells, where they liberate and accumulate cAMP. This messenger passes through intermediary steps across the cell to the luminal membrane, where the number of water channels (aquaporin 2) are increased. The luminal cell membrane is thus rendered water-permeable, which increases the renal water retention. The increased water reabsorption leads to a small, concentrated urine volume (antidiuresis), and a net gain of water that returns ECF osmolality towards normal. Initially, osmolality control overrides blood volume control.Fig. 24-6: Primary osmolality control of the renal water excretion. ADH and thirst systems maintain osmolality and ICV within narrow limits. Water overload decreases ECF osmolality and has the reverse effect, because the hypothalamic osmoreceptors suppress the ADH release, and the renal water excretion is increased already after 30 min (Fig. 24-6). When a person rapidly drinks one litre of water, the intestine absorbs water. Ions diffuse into the intestinal lumen and the blood osmolality falls causing a block of the ADH secretion (Fig. 24-6). Pure water is distributed evenly in all three body fluid compartments – just like intravenous infusion of one litre of 5% glucose in water. Intake of one l of isotonic saline implies ECV expansion, without dilution of body fluids. This expansion will not increase the urine volume much, so the increased ECV can be sustained for many hours. An intravenous infusion of one l of large dextran molecules (macrodex) stays mainly in the vascular space. 9. Regulation of renal sodium excretion In healthy persons, changes of blood volume (or ECV) or blood pressure control sodium excretion (Fig. 24-7). The dominating cation of the ECV is Na+ . The sodium intake is balanced by the sodium excretion as long as the thirst and other homeostatic systems are functional. During conditions where sodium intake exceeds renal sodium excretion, total body sodium and ECV increase. Conversely, total body sodium and ECV decrease, when sodium intake is lower than renal sodium excretion. This is because volume-pressor-receptors detect the size of the circulating blood volume (ECV) or pressure, and effector mechanisms adjust the renal sodium excretion accordingly. The volume-pressor-receptors are widely distributed. Low-pressure receptors are found in the pulmonary vessels and in the atria. An increased blood volume can also increase the arterial blood pressure and stimulate the well-known high-pressure baroreceptors in the carotid sinus and the aortic arch. Increased arterial pressure reduces sympathetic tone – also in the kidneys, whereas decreasing arterial pressure enhances sympathetic tone and renal salt retention. Arterial pressure receptors are also located in the renal preglomerular arterioles. Both stimuli in Fig. 24-7 release renin from macula densa, whereby angiotensin II and aldosterone is secreted (both sodium retaining hormones). A decrease in circulating blood volume leads to a decrease in NaCl delivery to the macula densa and release of the renin cascade. Conversely, an increase in circulating blood volume with increased NaCl delivery to the macula densa suppresses renin release and increases sodium excretion (Fig. 24-7).Fig. 24-7: Primary blood volume-pressure control of the renal Na+ -excretion. The effective circulating blood volume is protected – also during shock (Na+ -retention) and during hypertension (natriuresis). Increased salt intake increases blood volume and leads to natriuresis, possibly augmented by release of ANP (see below), nitric oxide and other factors. The excretion of Na+ depends upon several effector mechanisms out of which three are classical: The first factor is the glomerular filtration rate (GFR), which is responsible for the size of the filtered flux of Na+ across the glomerular barrier in the kidneys. Renal prostaglandins, generated in response to angiotensin II, are involved in maintaining the filtered flux of Na+.The second factor is the renin-angiotensin-aldosterone cascade (Fig. 24-5). The third factor consists of peptides with natriuretic effects. The most well-known peptide is called atrial natriuretic peptide (ANP) and originates from granules of the atrial myocytes. A low circulating blood volume with low atrial pressure increases renal sympathetic tone, reduces the stimulus of the low-pressure receptors in the atrial wall and thus the ANP secretion. Hereby, the natriuresis is reduced. - Renal natriuretic peptide or urodilatin from the distal tubule cells is related to ANP. Urodilatin has been isolated from human urine and contains four amino acids more than ANP. An increase in effective circulating blood volume, increases atrial pressure, reduces sympathetic tone and releases ANP and urodilatin leading to increased natriuresis. The main purpose of these mechanisms is to maintain an effective circulating blood volume by an increase or a decrease of the renal excretion of Na+. Initially, osmolality control is dominating. Finally, after a dangerous reduction in blood volume, volume-pressure receptors override the hypothalamic osmoreceptors and stimulate the ADH release and thirst. In the terminal phase, the body protects effective circulating blood volume at the expense of ECF osmolality. This paragraph deals with 1. Dehydration, 2. Overhydration, 3. Hyponatraemia, 4. Hypernatraemia, 5. Hypokalaemia, and 6. Hyperkalaemia. Dehydration is an abnormal reduction of the major fluid volumes (total body water with shrinkage of ECV). When we lose more than 5% of the total body water it has clinical consequences. The condition is life threatening if the patient loses 20 %. Accidents and surgery with a period of water deprivation, imply a rise in ECF osmolality and thus stimulation of both thirst and the hypothalamic osmoreceptors, whereby ADH is released. - Symptoms and signs of dehydration are thirst, dry mucous membranes, and decreased skin elasticity or turgor due to loss of ISF. Loss of effective circulating blood volume implies a low blood pressure in both the venous and the arterial system. Loss of more than one litre of ECV causes postural hypotension with dizziness, confusion and cerebral failure. Empty veins and cold skin characterise the peripheral venoconstriction. Finally, there is extreme tachycardia, which turns into terminal bradycardia and an arterial blood pressure that approach zero. Loss of salt and water frequently develops into hypo-osmolal dehydration (Fig. 24-8). This is because the thirst forces the patient to drink (salt free) water. Water dragged into the cells further reduces the hyposmolal ECV (Fig. 24-8). The small ECV elicits a hyperaldosteronism, which is called secondary, because it is not initiated as primary hypercorticism in the adrenal cortex. A precise compensation of the water loss results in pure hyponatraemia, where water eventually is drawn from ECV into the cells. The low [Na+] around the swelling cells reduces the potential gradient across the cell membranes with increased neuromuscular irritability (muscular twitching) and cardiac arrhythmias. Isosmolal dehydration is a proportional loss of water and solutes. There is no concentration gradient over the cell membranes, and the loss is mainly from ECV (Fig. 24-8).Fig. 24-8: Dehydration (hyperosmolal, isosmolal and hyposmolal). Hyperosmolal dehydration occurs in persons deprived of water. The hyperosmolal ECV drags water from ICV and dehydrates the cells (Fig. 24-8). This is intracellular dehydration.The hyperosmolality liberates ADH to restrict the water loss. The patient excretes a very small urine volume. Persons deprived of water at sea may drink seawater. Sea water is hypertonic saline and the victims die faster. When hypertonic saline reaches the ECV it aggravates the intracellular dehydration simultaneously with an extracellular overhydration. Intracellular dehydration leads to respiratory arrest and death of thirst. Overhydration is an abnormal increase of total body water - in particular ECV, and thus salt accumulation. The increase in the interstitial fluid volume is called oedema. Overhydration frequently occurs among patients in fluid therapy (ie, overhydration of iatrogenous origin).Increased salt intake by mouth is compensated by increased salt excretion by normal kidneys. However, a large saline infusion (0.9% NaCl) will expand ECV and total body water (isosmolal overhydration in Fig. 24-9). Inappropriately large infusions of saline lead to iatrogenous hyperosmolal overhydration, if they lose more water than salt (Fig. 24-9). Hyperosmolality drags water from the cells, so that the patient develops intracellular dehydration with hallucinations, loss of consciousness and eventually respiratory arrest. The patient with hyposmolal overhydration is typically in fluid treatment and develops muscle cramps and disorientation. The skin turgor is normal. A low serum - [Na+] confirms the diagnosis. The water overload in ECV is dragged into the cells in hyposmolal overhydration until osmolality balance (Fig. 24-9). In the brain and the muscles this intracellular overhydration causes headache, disorientation, increased spinal pressure, coma and muscle cramps. Both hyposmolal and hyperosmolal intracellular overhydration conditions are characterised by cerebral symptoms and signs. Fig. 24-9: Overhydration (hyperosmolal, isosmolal, and hyposmolal). Acute renal failure with decreased GFR reduces the flux of filtered NaCl (first factor) and thus the Na+ -excretion.Oedema is a clinical condition where the interstitial fluid volume (ISF) is abnormally large. A voluminous ISF is usually due to increased hydrostatic venous pressure (heart insufficiency), or a reduced colloid osmotic pressure (hypoproteinaemia) as predicted from Starlings law for transcapillary transport. Reduced protein synthesis (liver disease) and abnormal protein loss with the urine (proteinuria) causes hypoproteinaemia. Thus protein-losing kidneys are involved. Capillary damage (allergy, burns, inflammation etc) with increased capillary permeability causes local oedema. – Obstruction to lymphatic drainage can also cause oedema (scarring after radiation therapy, elephantiasis etc). Cardiac insufficiency with increased venous pressure and oedema formation increases sympathetic tone and thus releases the renin-angiotensin-aldosterone cascade (Fig. 24-5) causing Na+ -retention.Hepatic cirrhosis activates the cascade in a similar way - possibly including the release of nitric oxide. Hypoalbuminaemia reduces the colloid osmotic pressure of plasma, whereby water is distributed from the vascular space to the ISF. The fall in effective circulatory volume activates the renin cascade and leads to Na+ -retention. NSAIDs can activate the renin-angiotensin-aldosterone cascade, and the increased aldosterone leads to Na+ -retention and overhydration.Angiotensin II-receptor antagonists and ACE-inhibitors are utilized clinically to block the effects of angiotensin II in congestive heart failure, diabetes mellitus and hypertension. Blockade of the cascade reduces both preglomerular and postglomerular resistances.The supine position at bed rest increases venous return. This implies an increased cardiac output (Starlings law), a reduced ANF secretion from the atrial walls and a reduced renin-angiotensin-aldosterone cascade. This is why bed rest is beneficial for disorders with salt accumulation. Hyponatraemia (ie, plasma-[Na+] below 135 mM) is associated with dehydration, overhydration or normohydration (ie, a normal ECV and total body sodium content). Hyponatraemia with reduced ECV (ie, salt-deficient hyponatraemia) is caused by a salt loss in excess of the high water loss (ie, hyposmolal dehydration in Fig 24-10). This is seen in any type of hypoadrenalism including the rare primary hypoadrenalism (Addison’s disease). In Addison’s disease the entire adrenal cortex is destroyed by autoimmune reactions (80%) or by malignancy or infection. All three types of hormones are insufficiently produced (mineralocorticoids, glucocorticoids and sex hormones). The lack of aldosterone leads to Na+ -excretion and K+-retention with hyponatraemia combined with hyperkalaemia resulting in dehydration and hypotension.Hyponatraemia is developed in the following way (Fig. 24-10): 1. The first step is the salt loss in excess of the water loss. 2. Since the ECF-[Na+] is low, the ADH secretion is suppressed, and the water excretion is increased. Hereby, both the ISF and the vascular spaces are reduced often by more than 10%. 3. This is an adequate stimulus for the volume-pressure receptors, which override the osmoreceptors, whenever the effective circulatory volume is threatened. Fig. 24-10: The three body fluid compartments in a patient with salt-deficient hyponatraemia. The volume-pressure receptors stimulate both thirst and the release of ADH. The effective circulating volume is protected at the expense of osmolality! Still the blood pressure is falling, which impairs cerebral perfusion, causing confusion, headache and coma. The hyponatraemia implies a reduced resting membrane potential and thus a low threshold for neuromuscular stimulation resulting in muscle cramps. The large renal loss is seen with osmotic diuresis (hyperglycaemia and uraemia), excessive use of diuretics, renal tubular reabsorption defects, adreno-cortical insufficiency as aldosterone-antagonist-intoxication or other types of hypoaldosteronism. The extra-renal loss is often large from excessive sweating, diarrhoea, haemorrhage, vomiting, loss with ascites or bronchial secretion, and transudation from cutaneous defects. Normal kidneys normally compensate extra-renal loss. The urinary excretion of salt and water falls in response to volume depletion, so the urine is concentrated - but with less than 10 mM Na+. Normal sweat is a hypotonic solution, because Na+ is reabsorbed in the duct system. The [Na+] can increase up to 80 mM with increasing sweat flow - due to the limited time for the aldosterone-controlled Na+-reabsorption. Increased salt intake by mouth or intravenously is required as a supplement to the treatment directed at the primary cause. Low plasma- [Na+] in a chronically salt-deficient patient suggests a high aldosterone secretion from the adrenal zona glomerulosa. Further administration of aldosterone therefore may not have any effect. Hyponatraemia with increased ECV (water-excess hyponatraemia) is often caused by cardiac, hepatic, and renal insufficiency or by hypoalbuminaemia - see hyposmolal overhydration (Fig. 24-9). Hyponatraemia with normal ECV is often caused by stress (surgery, psychogenic polydipsia), abnormally high ADH release (in the syndrome of inappropriate antidiuretic hormone secretion, and in vagal neuropathy), increased sensitivity to ADH by drugs such as chlorpropamide and tolbutamide, or by intake of ADH-like substances (oxytocin). Pseudo-hyponatraemia is characterised by a spuriously low plasma value measured conventionally in the total volume of plasma, which includes an extra volume in cases with hyperlipidaemia or hyperproteinaemia etc. Plasma osmolality or plasma-Na+ measured with ion selective electrodes is the choise and the direct read value is normal. This is because Na+ is confined to the aqueous phase. Treatment of artefactual hyponatraemia (taking blood from an extremity into which isotonic glucose is infused) is also unnecessary.The normal plasma-[Na+] is 135-145 mM, and values above 170 mM are rare. Excessive infusion of saline (0.9% NaCl or 154 mM) can lead to hypernatraemia. Such alarmingly high levels create an emergency situation, where glucose infusion is indicated initially in order to reduce the high level slowly. The increased plasma osmolality elicits a strong desire to drink. The cause is sometimes water deficit due to pituitary diabetes insipidus, or to nephrogenic diabetes insipidus, where ingestion of nephrotoxic drugs have made the renal collecting ducts resistant to ADH. – Osmotic diuresis also causes water deficit with hypernatraemia just as excessive loss of water through the skin or lungs. Primary hyperaldosteronism (Conns disease) and all types of secondary hyperaldosteronism also lead to hypernatraemia combined with hypokalaemia and enlarged blood volume. Cerebral failure and convulsions are alarming signs, but there are no specific symptoms and signs of hypernatraemia. Polyuria, polydipsia and thirst suggest diabetes. Diabetes mellitus is easy to diagnose, and diabetes insipidus shows a low urinary osmolality. Pituitary diabetes insipidus is treated with an analogue of ADH (desmopressin, with a low pressor-effect).The normal potassium ion concentration in blood plasma is 3.5-5 mM. Hypokalaemia is caused by renal or extra-renal K+ -loss or by restricted intake. Long standing use of diuretics without KCl compensation is a frequent cause of hypokalaemia. Hyperaldosteronism (increased aldosterone secretion) is another cause. Vomit fluid only contains 5-10 mM of K+. Still, prolonged vomiting develops into hypokalaemia, because the Na+-loss stimulates the aldosterone secretion, which increases K+-excretion in the kidneys. Profuse diarrhoea causes marked hypokalaemia, also because the diarrhoea fluid contains up to 50 mM of K+. Hypokalaemia is seen in cardiac patients receiving digoxin treatment. Digoxin toxicity is imminent, because digoxin firmly binds to myocardial cells in hypokalaemia. Treatment must be directed towards the underlying cause. Infusion of potassium -rich fluid is dangerous, because of the marginal distance to hyperkalaemia.The reduced extracellular K+ hyperpolarises the cell membrane (increases the negativity of the voltage across the membrane). This reduces the excitability of neurons and muscle cells. Thus, hypokalaemia can result in muscle weakness and paresis. Hypokalaemia is associated with an increased frequency of cardiac arrhythmias with atrial and ventricular ectopic beats in particular in patients with cardiac disease . - Hypokalaemia inhibits release of adrenaline, aldosterone and insulin. Acute hyperkalaemia (ie, plasma-[K+] above 5 mM) is a normal condition following severe exercise, and normal kidneys easily eliminate K+ . In disease states the causes are insufficient renal excretion or increased release from damaged body cells as during long lasting hunger, exercise or in severe burns. A plasma- [K+] above 7 mM is life threatening due to asystolic cardiac arrest. Long term intake of b-blocking drugs, which inhibit the Na+-K+-pump, leads to hyperkalaemia that is accentuated by exercise. Hyperkalaemia reduces the size of the resting membrane potential (reduces the negativity of the voltage), whereby the threshold for firing is approached in neurons and striated muscle cells. The increased excitability in hyperkalaemia results in muscle contractions, cramps followed by muscle weakness. Hyperkalaemia leads to decreased cardiac excitability, hypotension, bradycardia and eventual asystole. The ECG is characterised by increased duration of the QRS-complexes and tented T-waves due to abrupt Ca2+-influx, contraction, and abrupt Ca2+ -binding (Fig. 24-3). Cardiac arrest occurs as ventricular fibrillation (the heart can never produce smooth tetanus) or as asystole. Insulin is used to drive K+ back into the cells - either by insulin infusion or by glucose infusion in order to release more insulin from the pancreatic islets. Usually, a combined glucose-insulin drop is applied. Other hormones (adrenaline, aldosterone) also stimulate the Na+-K+-pump and thereby increase cellular K+ -influx (Fig. 24-3) · The indicator dilution method: The indicator n mmol distributes in V litres of distribution volume. We measure the concentration Cp in mM, following even distribution, and calculate the volume, V: Eq. 24-1: V = n/Cp. (litre = mmol/(mmol/l) · When the tracer is radioactive potassium and thus distributed evenly in the exchangeable potassium pool, its specific Activity (SA) must be the same in urine, plasma or elsewhere in the pool. Eq. 24-2: Exchangeable body potassium = (Injected - eliminated)/SA. (Mol = Bq/(Bq per mol) We know the specific activity for the tracer (SA Bq per mol) from the plasma measurements. In this way we measure the exchangeable body potassium. The normal values are 41 mmol 39K per kg body weight for females, and 46 mmol per kg for males. · The following concentrations are found in normal plasma: [Na+] 135-145, [K+] 3.5-5, [Cl-] 96-106, [bicarbonate] 24, and total-[Ca2+] 2.5 mM. · The concentration of low molecular ions in the ultrafiltrate is affected by the Donnan effect (normally 5% for monovalent ions), and by the fractional content of water in plasma (0.94 normally): Eq. 24-3: [Low molecular ions] = Plasma conc. * Donnan factor/ 0.94. [Na+] = 141× 0.95/0.94 = 143 mmol/l of ultrafiltrate. Based on the Donnan effect alone, this result is less than 141. The Donnan effect on monovalent cations is simply more than compensated by the protein volume effect or fractional content of water in plasma (0.94). [Cl-] = 103×1.05/0.94 = 115 mmol/l of ultrafiltrate. Based on the Donnan effect this result should be greater than 103 and the protein volume effect contribute further. Such an ultrafiltrate is present in the kidneys and in ISF. · The extracellular fluid volume (ECV) can be measured if all inulin molecules are collected in the urine over 10-15 hours after the inulin infusion stopped. Eq. 24-4: ECV = Amount of inulin excreted/(Cp/0.94). The inulin distribution volume is more or less identical to the ECV. · Concentration of molecules in the filtrate are calculated as follows: Eq. 24-5: Cfiltr = Cp×Ffree/0.94 (mmol per l of ultrafiltrate). This value depends upon the fractional content of water in plasma (Fwater = 0.94 l of water per l of plasma) and of the fraction of free, unbound molecules (Ffree). For uncharged, free molecules like inulin Ffree is 1, and for protein-bound molecules Ffree is lower than 1 Each of the following statements has True/False options: A. Hyponatraemia with normal ECV is often caused by stress, abnormally high ADH release, increased sensitivity to ADH by drugs, or by intake of ADH-like substances.B. The total water content of a healthy person is 60%, and an extremely obese adult contains relatively more water. C. Hyponatraemia is defined as a plasma-[Na+] below 145 mM. D. A plasma- [K+] above 4.5 mM is life-threatening. E. An infusion of one l of 5% glucose is distributed evenly into all three compartments just as pure water. An infusion of one l of saline remains mainly in the ECV, whereas an infusion of one l of macrodex stays mainly in the vascular space. A healthy male with a body weight of 70 kg has a normal extracellular osmolality (300 mOsmol kg-1), and a normal ICV/ECV of 28/14 kg or l of water.One day he is the victim of severe burns and he suffers a water loss of 2.5 l of water (the salt loss is covered). 1. Calculate the new ECV osmolality following the water loss. 2. Does this hyperosmolality have consequences? 3. Following total restitution of the water compartments the patient undergoes surgery with skin grafts. During the long procedure he receives sufficient water by glucose infusion, but he looses 900 mOsmol NaCl. Calculate the new osmolality.4. Is it dangerous for a healthy individual to lose 6 kg of water without solutes? 5. Is it dangerous for a healthy individual to lose 6 kg of water as an isosmolal fluid from the ECV? A female patient (age 22 years; weight 71 kg) is in hospital suspect of potassium imbalance. She has taken diuretics for 2 years. She is tired and sleepy; her legs are paretic. The ECG shows prolongation of the Q-T interval, depression of the S-T segments and flattening of the T-waves. Her blood pH is 7.57 and the serum K+ -concentration is 2.9 mM. One morning she receives an intravenous injection of a solution containing the radioactive isotope of potassium (555,000 Becquerel, Bq, of 42K+ with a physical half-life of 12 hours). Following the injection her urine is collected in two periods (0-12 and 12 -24 hours). The first urine collection contained 40 mmol K+ (39K+) and 4144 Bq 42K+. The second urine specimen contained 40 mmol K+ and 2220 Bq 42K+. Both urine specimens were analysed for radioactivity exactly 24 hours after the injection, where the specific activity of her plasma was 55.5 Bq/mmol. The 42K+, retained after the first 12 hours, distribute in her body just like all other exchangeable K+. The body contains traces (0.012% of the total) of naturally occurring radioactivity (40K) with a half-life of 1.3 × 109 years. 1. Calculate the exchangeable K+ pool of her body after the 12‑hour distribution period. - Is the result normal? 2. Calculate the elimination rate constant (k) for exchangeable K+ in her body, and the biological half-life for this K+ in hours. Calculate the ratio between the physical and the biological half-life of K+. 3. What is the cause of her disease? 4. Describe the actions of diuretics. 5. Describe a method for measurement of her total body potassium. This case requires knowledge of the renal function (Chapter 25). Two groups of substances are evenly distributed in the ECV of a healthy 25-year-old man. His weight is 70 kg, and his extracellular volume (ECV) is 14 L. Both groups of substances disappear solely by excretion through the kidneys. His GFR is 120 ml/min, and his renal plasma flow (RPF) is 700 ml/min. 1. Inulin is representative for one family of substances. Inulin is only ultrafiltered in the kidneys. What fraction (k1) of the total amount of inulin in the body is maximally excreted in the urine per min? 2. The other substances are not only ultrafiltered, but they are also undergoing tubular secretion to such an extent that they totally disappear from the blood during the first passage. What is the elimination rate constant (k2) for these substances? Try to solve the problems before looking up the answers. · Water permeable membranes separate the three body fluid compartments, so that they contain almost the same number of osmotically active particles (expressed as mOsmol per kg of water or the same freeze-point depression). The three compartments are the intracellular fluid volume (ICV), the interstitial fluid volume (ISV) and the vascular space. · The sum columns of electrolyte equivalents in muscle cells are essentially higher than the extracellular sum columns, because cells contain proteins, Ca2+, Mg2+ and other molecules with several charges per particle. · Females contain less water as an average compared to males. Such differences show sex dependency, but the important factor is the fraction of body fat, since fat tissue contains significantly less water than other tissues (only 10%). Sedentary, overweight persons contain 50-55 % water dependent on the body fat content, and regardless of sex. · Primary hyperaldosteronism (Conns hypercorticism disease) and all types of secondary hyperaldosteronism also lead to hypernatraemia combined with hypokalaemia and enlarged blood volume. Cerebral failure and convulsions are alarming signs, but there are no specific symptoms and signs of hypernatraemia. · Polyuria, polydipsia and thirst suggest diabetes insipidus and low urinary osmolality is a clear indication. Pituitary diabetes insipidus is treated with an analogue of ADH (desmopressin, with a low pressor-effect). · Regulation of K+-balance: The daily intake of K+ is matched by the renal K+-excretion and our daily urine contains 2-5 g of K+. · Acid-base balance. The pH of the ICV and the ECV is maintained within narrow limits (many metabolic processes are sensitive to pH). The acid-base balance is accomplished by co-operative action of the kidneys and the lungs. · Hypokalaemia reduces the excitability of neurons, muscle cells and the myocardial syncytium. Thus, hypokalaemia can result in muscle weakness, paresis, and cardiac arrhythmias with ectopic beats and cardiac arrest in diastole. · Hyperkalaemia increases the excitability of neurons, muscle cells and the myocardium. · Acute hyperkalaemia is a normal condition following severe exercise, and normal kidneys easily eliminate this. In disease states the causes of hyperkalaemia are insufficient renal excretion or increased release from the body cells as during long lasting hunger. · A plasma- [K+] above 7 mM is life threatening due to ventricular fibrillation or cardiac arrest in systole. Tented T-waves and increased QRS-complexes characterise the ECG. Astrup, P, P. Bie, and H.C. Engell. ‘Salt and water in culture and medicine.’ Munksgaard, Copenhagen, 1993. Knox, F. G. “Physiology of potassium balance.” Am.J. Physiol. 275 (Adv. Physiol. Educ. 20): S142-S147, 1998.
|
||
Click here to introduce your comments or contributions