New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 25: Renal Physiology and Disease
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 25
|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
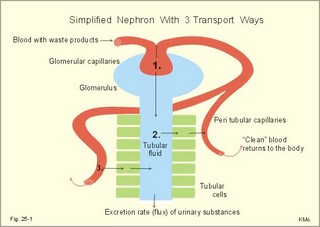
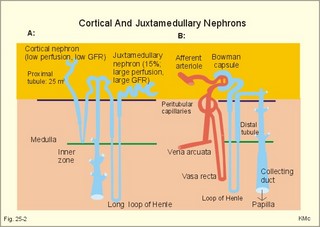
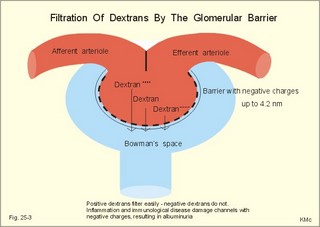
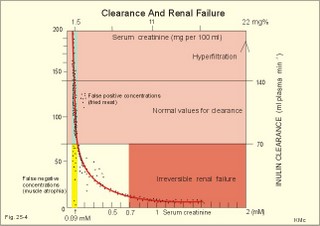
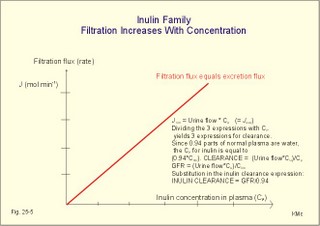
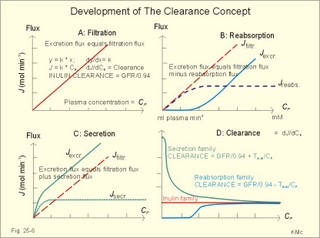
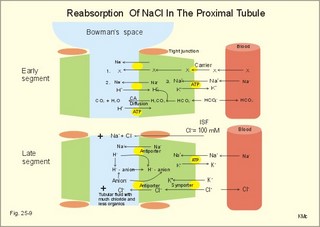
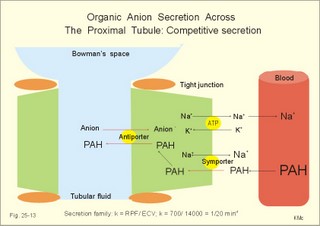
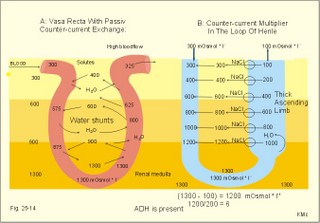
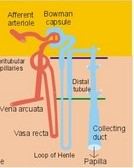
· To define the concepts: Nephron, glomerular filtration, tubular secretion and reabsorption, renal lobulus, renal plasma clearance, osmolar clearance, tubular passage fraction, reabsorption fraction, excretion fraction, filtration fraction, plasma extraction fraction, proximal and distal system, glomerular propulsion pressure, net filtration pressure, renal threshold, and the maximal transfer (Tmax) for tubular secretion and reabsorption. · To describe the renal circulation and measurement of renal bloodflow, a superficial and a juxtamedullary nephron, the juxtaglomerular apparatus, and the concentrating mechanism of the kidney. · To calculate the relation between half-life, elimination rate constant, clearance and distribution volume of a substance treated in the kidneys. · To explain the normal renal function including the control functions, use of endogenous creatinine clearance as a renal test, the renal treatment of the filtration- reabsorption- and secretion-families of substances, the glomerular filtration rate (GFR), the angiotensin-renin-aldosterone cascade, the tubulo-glomerular feedback, the proximal and distal transport processes, and micturition. To explain the pathophysiology of common renal disorders including renal oedema. · To use the above concepts in problem solving and in case histories. · The glomerulus and the proximal tubule are responsible for filtration of plasma and for major reabsorption of water and solutes. Glomerular filtration is due to a hydrostatic/colloid osmotic pressure gradient. · Tubular reabsorption is the movement of water and solute from the tubular lumen to the tubule cells and often further on to the peritubular capillary network. · Tubular secretion represents the net addition of solute to the tubular fluid in the lumen. · All substances treated by the kidneys can be divided into three groups or families, namely the filtration group, the reabsorption group and the secretion group. · Anuria refers to a total stop of urine production frequently caused by circulatory failure with anoxic damage of the tubular system. · (Renal plasma) Clearance is a cleaning index for blood plasma passing the kidneys. The efficacy of this cleaning process is directly proportional to the excretion rate for the substance, and inversely proportional to its plasma concentration. · Diuresis is an increased urine flow (ie, volume of urine produced per time unit). · Excretion fraction (EF) for a substance is the fraction of its glomerular filtration rate, which passes to and is excreted in the urine. · Extraction fraction (E) for a substance is the fraction extracted by glomerular filtration from the total amount of substance delivered to the kidney during one passage of the arterial blood plasma. · Free water clearance is the difference between urine flow and osmolar clearance (see below). The free water clearance is an indicator of the excretion of solute-free water by the kidneys. Excess water is excreted compared to solutes, when free-water clearance is positive. Excess solutes are excreted compared to water, when free-water clearance is negative. – Free water clearance is an estimate of the renal capacity for excretion of solute-free water. · Glomerular filtration is due to a hydrostatic/colloid osmotic pressure gradient - the Starling forces. · Glomerular filtration fraction (GFF) is the fraction of the plasma flowing to the kidneys that is ultrafiltered (GFR/RPF). GFF is normally 0.20 or 1/5. - The GFF is reduced during acute glomerulonephritis. · Glomerulonephritis is an autoimmune injury of the glomeruli of both kidneys. · Glomerular filtration rate (GFR) is the volume of glomerular filtrate produced per min. · Glomerular propulsion pressure in the blood of the glomerular capillaries is the hydrostatic minus the colloid osmotic pressure of the blood (ie, 2-3 kPa in a healthy resting person). · Glomerulo-tubular balance refers to the simultaneous increase in NaCl and water reabsorption in the proximal tubules as a result of an increase in GFR and filtration rate of NaCl. An almost constant fraction of salt and water is thus reabsorbed regardless of the size of GFR. · Nephron: A nephron consists of a glomerulus, a proximal tubule forming several coils (pars convoluta) before ending in a straight segment (pars recta), the thin part of the Henle loop and a distal tubule also with a pars recta and a pars convoluta. · The nephrotic syndrome refers to a serious increase in the permeability of the glomerular barrier to albumin, resulting in a marked loss of albumin in the urine. The albuminuria (more than 3 g per day) causes hypoalbuminaemia and generalized oedema. · Net ultrafiltration pressure is the pressure gradient governing the glomerular filtration - the net result of the so-called Starling forces (see Fig. 25-7). · Osmolar clearance is the plasma volume cleared of osmoles (solutes) each minute. – Osmolar clearance is also defined as the fictive urine flow that would have rendered the urine isosmolar with plasma. - Osmolar clearance is the difference between the urine flow and the free water clearance, and osmolar clearance estimates the renal capacity to excrete solutes. · Osmolarity is the amount of osmotically active particles dissolved in a litre of solution. · Proximal tubule consists of the proximal convoluted tubule and pars recta. · Renal threshold for glucose is the blood glucose concentration at which the glucose can be first detected in the urine (appearance threshold) or at which the reabsorption capacities of all tubules are saturated (saturation threshold). · Renal ultrafiltrate is also compared to plasma water, because it is composed like plasma minus proteins. The fraction of one litre of plasma that is pure water is typically 0.94. Thus, the concentration of many substances in the ultrafiltrate, Cfiltr, is equal to Cp/0.94. · Single effect gradient is a transepithelial concentration gradient between the tubular fluid and the medullary interstitial fluid established at each level of the thick ascending limb by active NaCl reabsorption. · Tmax refers to the maximal net transfer rate of substance by tubular secretion or reabsorption. · Tubular passage fraction. The fraction of the amount ultrafiltered of substance passing a cross section of the nephron is the passage fraction. The passage fraction for inulin does not vary at all throughout the nephron. The passage fraction for inulin is one and remains so. · Tubular reabsorption fraction. The reabsorption fraction is the reverse of the passage fraction (1 minus the passage fraction). · Tubular reabsorption (active or passive) is the net movement of water and solute from the tubular lumen to the tubule cells and often further on into the peritubular capillary network. · Tubular secretion (active or passive) represents the net addition of solute to the tubular lumen. · Tubulo-glomerular feedback (TGF) controls the glomerular capillary pressure and the proximal tubular pressure – thus stabilising delivery of solute and volume to the distal nephron. The macula densa-TGF mechanism responds to disturbances in distal tubular fluid flow passing the macula densa. - Renal autoregulation is caused by myogenic feedback and by the macula densa-TGF mechanism. This paragraph deals with 1. The nephron, 2. Clearance and three clearance families, 3. Ultrafiltration and the inulin family, 4. Tubular reabsorption and the glucose family, 5. Tubular secretion and the PAH family, 6. Water and solute shunting by vasa recta, 7. Concentration or dilution of urine, 8. Renal bloodflow, 9. Macula densa-tubulo-glomerular feedback, 10. Non-ionic diffusion, 11. Tests for proximal and distal tubular function, 12. Stix testing with dipstics, and 13. Diuretics. The kidneys transport substances by three vectorial processes. Vectorial processes are characterized by their direction and size only (Fig. 25-1).Fig. 25-1: Renal transport. Black arrows indicate three vectorial transporting processes in a nephron: 1. Glomerular ultrafiltration is caused by a hydrostatic/colloid osmotic pressure gradient (the Starling forces), 2. Tubular reabsorption is the net movement of water and solute from the tubular lumen to the tubule cells and to the peritubular capillaries, and 3. Tubular secretion represents the net addition of solute to the tubular fluid. The final excretion rate of the substance s in the urine is called net-flux, Js, in Fig. 25-1. 1a. Nephron anatomy The functional unit is the nephron. Each human kidney contains 1 million units at birth. Each nephron consists of a glomerulus (ie, many glomerular capillaries in a Bowman's capsule), a proximal tubule forming several coils (pars convoluta) before ending in a straight segment (pars recta), the thin part of the Henle loop and a distal tubule also with a pars recta and a pars convoluta. The distal tubule ends in a collecting duct together with tubules from several other nephrons. The kidney (average normal weight 150 g) consists of a cortex and a medulla. The medulla is composed of renal pyramids, the base of which originates at the corticomedullary junction. Each pyramid consists of an inner zone (the papilla) and an outer zone. The outer zone is divided into the outer medullary ray and the inner ray. The rays consist of collecting ducts and thick ascending limbs of the nephron. A kidney lobulus is a medullary ray with adjacent cortical tissue. A kidney lobule is a pyramid with adjacent cortical tissue. The loop of Henle is a regulating unit. Actually, the Henle loop consists of the proximal pars recta, the thin Henle loop and the distal pars recta, which ends at the level of macula densa. The thin descending limb contains a water channel (called aquaporin 1) in both the luminal and the basolateral membrane. The last segment of the thick ascending limb is called the macula densa. The juxtaglomerular (JG) apparatus include the macula densa and granular cells of the afferent and efferent arterioles. Granular cells are modified smooth muscle cells that produce and release renin. The distal tubule is convoluted from the macula densa of the JG apparatus (Fig. 25-2). The illustration shows a collecting duct, which receives urine from many nephrons. Several collecting ducts join to empty through the duct of Bellini into a renal cup or calyx in the renal pelvis. The superficial nephron (represented on the left side of Fig. 25-2 A) does not reach the inner zone of the medulla, because its loop of Henle is short. These small, cortical nephrons have a smaller blood flow and glomerular filtration rate (GFR) than the deep, juxtamedullary nephrons (which are located close to the medulla and comprise 15% of all nephrons). The total inner surface area of all the glomerular capillaries is approximately 50-100 m2. Mesangial and endothelial cells in the glomerulus secrete prostaglandins and exhibit phagocytosis. Many vasoconstrictors contract the mesangial cells, reduce the gomerular filtration coefficient (Kf – see later) and thus also GFR. The proximal tubules have an inner area of 25 m2 due to characteristic microvilli or brush borders (containing carboanhydrase). Fig. 25-2: A: A superficial and a deep, juxtamedullary nephron leading to the same collecting duct. B: A juxtamedullary nephron with related blood vessels. The juxtamedullary nephron has a long, U-shaped Henle loop. The bottom of this loop extends towards the tip of the papilla (apex papillae) at the outlet of the collecting duct (Fig. 25-2). The juxtamedullary nephrons have large corpuscles with relatively large bloodflow. These nephrons also receive blood through afferent arterioles with large diameters, and return blood through efferent arterioles with small diameters. When the blood has passed the juxtamedullary glomeruli it continues to a primary capillary network and to the vasa recta in the medulla. The blood collects in vena arcuata, vena interlobaris and finally into vena renalis. 1b. The glomerular barrier The filtration barrier of the glomerulus consists of capillary endothelium, basement membrane and the epithelial layer of Bowmans capsule consisting of podocytes with foot processes. The holes or fenestrae of the endothelium have a radius of approximately 40 nm (covered by a thin diaphragm) and are permeable to peptides and small protein molecules. The basement membrane consists of a network of fibrils permeable to water and small solutes. The podocytes cover the basement membrane with foot processes separated by gaps called split-pores through which the filtrate is retarded, because each split is covered by a membrane. All small ions and molecules with an effective radius below 1.8 nm (water, ions, glucose, inulin etc) filtrate freely. Substances with a radius of 1.8-4.2 nm are less filterable, and substances with a radius above 4.2 nm cannot cross the barrier. All channels of the glomerular barrier carry negatively charged molecules that facilitate the passage of positively charged molecules (eg, polycationic dextrans, Fig.25-3). Dextran macromolecules can be electrically neutral or they have negative (anionic) or positive (cationic) charges. Fig. 25-3: Filtration of dextran molecules across the glomerular barrier. The barrier contains glycoproteins with negative charges. Positive charged dextran molecules are attracted by the negative charges and filter easily. Positive charged molecules with an effective radius of 3 nm filter easier than negative charged molecules of the same size. These molecules can act as effective osmotic diuretics. Immunological or inflammatory damages of the glomerular barrier reduce the negative charge of the barrier. Hereby, negative protein molecules leave the plasma easier and proteinuria occurs in a number of glomerular disorders. 1c. Pregnancy and age The glomeruli grow and the size and weight of the kidneys increase during pregnancy, accompanied by increases in both renal bloodflow and filtration rate. The number of glomeruli and their tubules decrease with age. Drugs that are excreted by renal mechanisms can easily cause toxic accumulation in the elderly with poor kidney function. In 1926 Poul Brandt Rehberg, an associate of August Krogh, found the muscle metabolite creatinine extremely concentrated in human urine (CU mg per ml) compared to plasma (CP mg per ml). He also measured the urine flow (urine production per min). Thus, the concentration index, CU/CP, is large for creatinine. Multiplying this index with the urine flow yields a result greater than similar results derived for most other substances (Eq. 25-1). Brandt Rehberg used this concept (later termed clearance) as his measure of renal filtration rate. The work with these matters developed into the idea of a filtration-reabsorption type of kidney. Rehberg was the first to realise that the reabsorption in the proximal tubules controls the filtration. A few years later Rehberg´s renal filtration rate was called creatinine clearance and used as a measure of the glomerular filtration rate (GFR). The renal plasma clearance is a cleaning index for blood plasma passing the kidneys. The efficacy of this cleaning process is directly proportional to the excretion rate for the substance and inversely proportional to its plasma concentration (Eq. 25-1). Clearance is the ratio between excretion rate and plasma concentration for the substance. Renal clearance can also be thought of as the volume of arterial plasma completely cleared of the substance in the kidneys within one min, or the number of ml arterial plasma containing the same amount of substance as contained in the urine flow per minute (Eq. 25-1). 2a. Glomerular filtration rate The glomerular filtration rate, GFR, is the volume of glomerular filtrate produced per min. In healthy adults the GFR is remarkably constant about 180 l each day or 125 ml per min due to intrarenal control mechanisms. In many diseases the renal bloodflow, RBF, and GFR will fall, whereby the ability to eliminate waste products and to regulate body fluid volume and composition will decline. The degree of impaired renal function is shown by the measured GFR. GFR is routinely measured as the endogenous creatinine clearance. The endogenous creatinine production is from the creatine metabolism in muscles and proportional to the muscle mass. In a 70-kg person creatinine is produced at a constant rate of 1.2 mg per min (1730 mg daily). This production is remarkably constant from day to day, only slightly affected by a normal protein intake, and equal to the rate of creatinine excretion. Both the serum creatinine and the renal creatinine excretion fluctuate throughout the day. Therefore, it is necessary to collect the urine for 6-24 hours and measure the creatinine excretion rate (ie, the urine flow rate multiplied by the creatinine concentration in the urine). A single venous blood sample analysed for creatinine in plasma is all that is needed to provide the endogenous creatinine clearance (Eq. 25-1). Theoretically, two small errors disturb the picture, but both are overestimates. At the normally low plasma concentrations of creatinine, a modest tubular secretion of creatinine from the blood is detectable resulting in up to 15% overestimation of the creatinine excretion flux. Most laboratories measure creatinine in serum instead of plasma, which results in an overestimation of plasma creatinine. Thus, calculation of a fraction with both an overestimated nominator and denominator results in a value close to that of GFR in almost all situations, where the renal function is near normal. With progressive renal failure the plasma creatinine rises, and the creatinine secretion increases the nominator in the clearance expression even more, so the measured clearance will overestimate GFR. Still, the clearance provides a fair clinical estimate of the renal filtration capacity (GFR). In most cases a normal creatinine clearance (above 70 ml plasma per min at any age) is comparable with the normal range for serum creatinine (around 0.09 mM in Fig. 25-4). The serum creatinine concentration is inversely proportional to the creatinine clearance, and also a good estimate of GFR. Renal failure is almost always irreversible, when the serum creatinine is above 0.7 mM. Fig. 25-4: Creatinine clearance versus serum creatinine. – A low serum creatinine indicates normal kidney function, but not always (see false negative concentrations). – An elevated serum creatinine indicates kidney failure, but not always (see false positive concentrations). Serum [creatinine] and serum [urea] depend upon both protein turnover and kidney function. The serum [creatinine] and [urea] are large after intake of meals extremely rich in (fried) meat, although the kidney function is normal (false positive concentrations in Fig. 25-4). In some materials up to 15% of measured serum creatinine concentrations are normal, although the kidney function fails (false negative values in Fig. 25-4). Long-term hospitalisation often leads to muscular atrophy, which reduces creatinine production and excretion. The serum creatinine concentration is maintained normal because of a similar fall in kidney function (GFR). Half the osmolality of normal urine is due to urea, and the other half is mainly due to NaCl. The osmolarity of urine varies tremendously (from 50 to 1400 mOsmol per l). Physiological changes of the renal bloodflow often parallel changes of GFR. A reduced GFR implies a smaller tubular Na+-reabsorption and thus a smaller O2 demand. When kidneys are perfused by anoxic blood the tubular reabsorption is blocked first, and then the GFR is reduced. As tubular Na+ -reabsorption is the main oxidative energy demanding activity, a high GFR is correlated to high oxygen consumption in the normal kidney. The size of GFR is determined by the factors shown in Fig. 25-7. The resistance of the glomerular barrier is extremely small in healthy human kidneys. 2b. Inulin Inulin is the ideal indicator for determination of GFR, because of the following three relations: 1. Inulin is a polyfructose (from Jewish artichokes) without effect on GFR. Inulin has a spherical configuration and a molecular weight of 5000. Inulin filters freely through the glomerular barrier. Inulin is uncharged and not bound to proteins in plasma. Inulin crosses freely most capillaries and yet does not traverse the cell membrane (distribution volume is ECV). Since one litre of plasma contains around 0.94 l of water, the ultrafiltrate concentration of inulin is Cp/0.94. 2 All ultrafiltered inulin molecules pass to the urine. In other words, they are neither reabsorbed nor secreted in the tubules. Inulin is an exogenous substance - not synthesised or broken down in the body. 3. Inulin is non-toxic and easy to measure. Thus, under steady-state conditions, the rate of inulin leaving the Bowman's capsulesmust be exactly equal to the rate of inulin arriving in the final urine. The main idea is to measure the amount of inulin excreted in the urine during a timeperiod were the plasma [inulin] is maintained constant by constant infusion of inulin. After one hour the subject urinates, and the urine volume and inulin concentration in the urine and plasma is measured. The amount of inulin filtered through the glomerular barrier per min is: (GFR × Cp/0.94). All inulin molecules remain in the preurine until the subject urinates. Thus, the amountexcreted is equal to the amount filtered and Eq. 25-4 is developed (see later). Since the inulin clearance is 180 l per 24 hours for young, healthy males or 125 ml per min, the GFR must be (125 × 0.94) = 118 ml per min. The inulin clearance is 10% lower for young females than for young males due to the difference in average body weight and body surface area. The normal values for both sexes decrease with age to 70 ml per min after the age of 70. Inulin clearance is a precise experimental measure and the ideal standard, but inulin must be infused intravenously, and the method is not necessary in clinical routine. If the clearance of a substance has the same value as the inulin clearance for the person, then the substance is only subject to ultrafiltration. Theoretically, reabsorption might balance tubular secretion and give the same result. If the clearance of a substance is greater than the inulin clearance, then clearly this substance is being added to the urine as it flows along the tubules; in other words, it is being secreted. Similarly, if the clearance of a substance is less than the inulin clearance, it means that the substance is being reabsorbed at a higher rate than any possible secretion. The extracellular fluid volume (ECV) can be measured with inulin as inulin does not pass the cell membrane (see Chapter 24 and Eq. 24-4). The elimination of inulin is exponential - ie, the fraction (k) of the remaining amount in the body that disappears per time unit is constant (see Chapter 1). Since the filtration family of substances is eliminated from the blood solely by filtration, the elimination depends only on GFR, and the distribution volume is that of inulin (ECV). Thus, the elimination rate constant (k= 0.69/T½) for the inulin family is roughly equal to (GFR*Cp)/(ECV*Cp). 2c. The three clearance-families All substances treated by the kidneys can be divided into three groups or families, namely the filtration-, the reabsorption-, and the secretion- family. The kidney treats the filtration family of substances (see later) just like inulin. The filtration rate (Jfiltr) for inulin equals the excretion rate (Jexcr), and both increase in direct proportion to the rise in Cp (Fig. 25-5). The clearance is the slope of the curve, and it is obviously a constant value that is independent of Cp. Fig. 25-5:The straight line shows a direct relationship between the filtration rate and the concentration for the inulin family of substances in plasma. The reabsorption or glucose family contains many vital substances (see later). For the reabsorption family of compounds, the excretion flux is equal to the filtration flux minus the reabsorption flux. The maximal reabsorption flux (Tmax) is reached above a certain threshold. Above this saturation threshold the clearance for the reabsorption family is equal to (the inulin clearance - Tmax/Cp), according to the mathematical argument in Fig. 25-8. The secretion or PAH family comprises endogenous substances and drugs (see later). Foreign substances are often distributed in the ECV, but some of them are also entering cells (ICV). At low concentrations their elimination rate constant (k) is roughly equal to renal plasma flow (RPF) divided by ECV: ( RPF*CP/ECV*CP) = RPF/ECV. Thus, k equals RPF/ECV or 1/20 min-1 in most healthy persons. The k value corresponds to a half-life of 14 min (T½ = ln 2/k).2d. Excretion rate and clearance. Excretion rate curves for inulin can be changed into clearance by a simple mathematical procedure: Differentiating the excretion flux curve for the inulin family with respect to Cp produce the renal plasma clearance curves for these substances. Let us assume that the curves are from a resting person in steady state with a normal inulin clearance (the slope of the line in Fig. 25-6,A). For the inulin family the excretion flux equals (urine flow × Cu), and by division with Cp we have the inulin clearance. Fig. 25-6: A, B, and C are the filtration-reabsorption- and secretion-families of substances, respectively. - D shows the clearance curves. For all substances belonging to the inulin family the excretion flux curves are linear, so the rate of change (which is the clearance) must be constant in a given condition (Fig. 25-6A). The results of the three excretion fluxes are plotted with Cp as the dependent variable (x-axis of Fig. 25-6, ABC). The excretion flux curves for the three families of substances, when differentiated (dJexcr/dCp), provide us with the three possible clearance curves (Fig. 25-6, D). For the reabsorption family, the clearance is zero at first, because the excretion is zero (Fig. 25-6 D). The clearance increases, and finally it approaches the inulin clearance. Therefore, the clearance is steadily increasing towards inulin clearance with increasing Cp. For the secretion family, the clearance must also be equal to the excretion flux divided by Cp. When the [PAH] increases, more and more PAH is eliminated by filtration, and the secretory elimination is relatively suppressed (so-called auto-suppression). The clearance for the secretion family is falling with increasing Cp, and approaches that of inulin (Fig. 25-6 D).
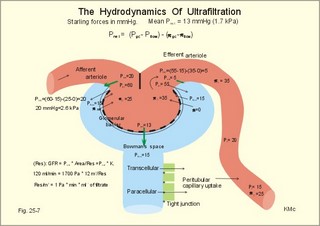
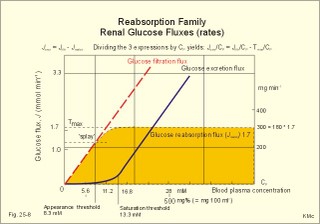
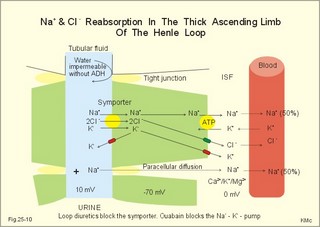
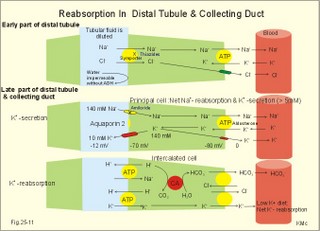
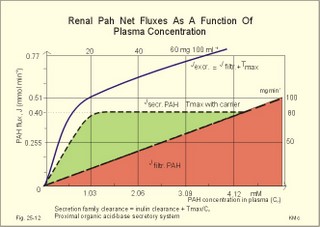
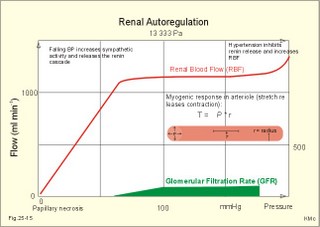
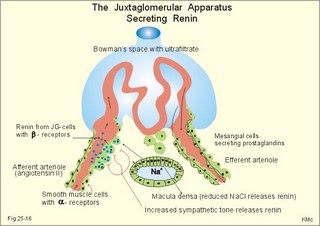
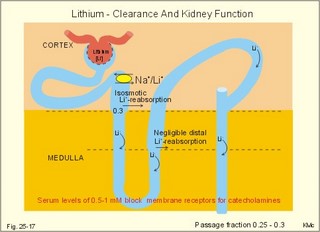
3. Ultrafiltation and the inulin family In a healthy person at rest almost 25% of cardiac output passes the two kidneys (1200 ml each min). The blood reaches the first part of the nephron through the afferent arteriole to the glomerular capillaries. In the glomerular capillaries the hydrostatic pressure is approximately 60 mmHg at the start and 55 mmHg at the end (Fig. 25-7). The inulin or filtration family consists of inulin, radioactive indicators( 51Cr-EDTA, 57Co- marked B12, 14C-marked inulin, 3H-marked inulin, iothalamate marked with 125I or 131I), mannitol, raffinose, sucrose, thiocyanate, and thiosulfate. These substances are more or less evenly distributed in the ECV.3a. The Starling forces The pressures governing the glomerular ultrafiltration rate (GFR) are called the Starling forces (see equation in Fig. 25-7). Normally, filtration continues throughout the entire length of the glomerular capillaries in humans, because the net ultrafiltration pressure (Pnet) is positive also at the efferent arteriole. The average values for determinants of GFR are given in the first equation of Fig. 25-7. The hydrostatic pressure gradient is an important determinant of GFR. The glomerular filtration coefficient is called Kf. The Kf is equal to the filtration surface area divided by the resistance of the glomerular barrier and thus a constant for a given barrier (Fig. 25-7). The value of Kf (also called the reciprocal glomerular hydrodynamic resistance) is reduced in diabetes, glomerulonephritis and hypertension. Vasoactive substances constrict or dilatate the glomerular mesangial cells and change the value of Kf. In other conditions, the forces opposing filtration become equal to the forces favouring filtration at some point along the glomerular capillaries. This is called filtration equilibrium. The hydrostatic pressure in Bowmans space below the glomerular barrier is about 15 mmHg or 2 kPa (PBow in Fig. 25-7). This pressure is almost equal to the proximal tubule pressure, since there is no measurable pressure fall along this segment. Fig. 25-7: Net ultrafiltration pressures in afferent and efferent end of glomerular capillaries. The Starling forces determine the final ultrafiltration pressure (Pnet) across the glomerular barrier. There is almost no colloid-osmotic pressure in Bowmans space, but an oncotic pressure of approximately 25 mmHg in the incoming plasma, mainly due to proteins, which are up-concentrated, when fluid leaves the the plasma for Bowmans space. Hereby, the protein-oncotic pressure (pgc) may increase from 25 to 35 mmHg at the end of the glomerular capillary (Fig. 25-7). The higher the renal plasma flow (RPF), the lower is the rise in pgc. A selective increase in the resistance of the afferent arteriole reduces both the RPF and the glomerular hydrostatic pressure (Pgc), but GFR decreases more than RPF, so the filtration fraction (= GFR/RPF) falls. In contrast, a rise in the resistance of the efferent arteriole reduces RPF but increases Pgc (Fig. 25-7). Instantly, GFR increases slightly, but GFR eventually decreases due to the rise in pgc. As RPF falls more than GFR the filtration fraction increases. A combined increase in both the afferent and the efferent arteriolar resistance (as caused by most vasoconstrictors) may also reduce RPF more than GFR, and increase the filtration fraction. 3b. The net ultrafiltration pressure The net ultrafiltration pressure (Pnet) varies from 20 to 5 mmHg through the glomerular capillaries, and provides the force for ultrafiltration of a fat- and protein- free fluid across the glomerular barrier into Bowmans space and flow through the renal tubules (Fig. 25-7). The ultrafiltrate is isosmolar with plasma, almost protein free, and contains low molecular substances in almost the same concentration as in plasma water. The proximal tubular reabsorption takes place through para- and trans-cellular pathways. In the peritubular capillaries, the Starling forces are seemingly adequate for capillary uptake of interstitial fluid (Fig. 25-7). The hydrostatic net pressure in the proximal tubules – and with it the GFR - is remarkably well maintained in spite of changes in proximal reabsorption of salt and water. An acute defect in the proximal reabsorption mechanism results in an initial rise in proximal hydrostatic pressure and the GFR is reduced. Due to autoregulation (see paragraph 9), the proximal hydrostatic pressure is rapidly normalised at a new steady state. Sympathetic stimulation increases both the proximal reabsorption rate and the peritubular capillary uptake (Fig. 25-7). Hereby, the hydrostatic pressure falls in the proximal tubules and Bowman's capsule so GFR may increase. In reverse, angiotensin II secretion inhibits the proximal reabsorption rate, increases the proximal pressure and may reduce GFR. The total distal flow resistance below the proximal tubules (ie, in the distal system) is large and important. The distal resistance has two major components namely a high resistance in the Henle loop and an even higher resistance in the remaining distal system including the collecting ducts.The resistance of the glomerular barrier is calculated in Fig. 25-7 to be extremely small. Normally, there is hardly any hydrodynamic resistance to glomerular ultrafiltration. 4. Tubular reabsorption and the glucose family The reabsorption or glucose family contains vital substances such as glucose, amino acids, albumin, acetoacetates, ascorbic acid, beta-hydroxybutyrate, carboxylate, vitamins, lactate, pyruvate, Na+, Cl-, HCO3-, phosphate, sulphate and urea.4a. Tubular handling of glucose Tmax is the maximum transfer or net reabsorption flux (Jreabs) for glucose (mol.wt. 180 g per mol) in the proximal tubules. The optimal value for this glucose transporter is 300 mg/min or 300/180 = 1.7 mmol/min for healthy, young subjects with a body weight of 70 kg. For the reabsorption family of substances, the excretion is zero at first since the entire filtered load is reabsorbed (all glucose is reabsorbed, see Fig. 25-8). The excretion flux increases then linearly with increasing filtration flux. Fig. 25-8: Renal Glucose rates as a function of the plasma concentration (Cp). The appearance threshold is the blood plasma [glucose] at which the glucose can be first detected in the urine (normally 8.3 mM or 150 mg%). This occurs when most but not all nephrons are saturated (Fig. 25-8). The actual saturation threshold, the point where all nephrons are glucose-saturated, is much higher (normally above 13.3 mM). The concentration difference (13.3 - 8.3 = 5 mM) represents a similar reabsorption rate difference (1.7 - 1.0 = 0.7 mmol/min at normal GFR) called splay. The reabsorption capacity for glucose in the proximal tubule cells becomes saturated at these high blood concentrations (Fig. 25-8). 4b. Urea transport The water reabsorption in the proximal tubules increases the urea concentration in the fluid. Since urea is uncharged and diffuses easily, it will diffuse passively to the peritubular capillary blood. The passage fraction at the outlet of the proximal tubule is around 0.5 (50% of the filtered load). Urea is thus reabsorbed in the proximal tubules and also in the inner medullary collecting ducts and secreted in the thin descending and ascending limb of the Henle loop (see later). The kidney reuses urea by recirculation in the intra-renal urea recycling circuit: Inner medullary collecting ducts – medullary interstitium – loop of Henle – distal tubules – collecting ducts. The net reabsorption flux is around 50% of the filtration flux at normal urine flow. The normal urea concentration in plasma is 5mM, and the excretion flux for urea is proportional to this urea concentration. 4c. Proximal tubular reabsorption Healthy proximal tubules reabsorb approximately 70% of the filtered water, Na+, Cl-, K+ and other substances. The tubular passage fraction for these substances at the outlet of the proximal tubule is 0.3 (30%). The reabsorption of fluid is isosmotic. Almost all filtered glucose, peptides and amino acids are also reabsorbed by the proximal tubules. The Cl- reabsorption is passive. This ion follows the secondary active reabsorption of Na+ in order to maintain electrical neutrality. Reabsorption of water is passive as a result of the osmotic force created by the reabsorption of NaCl. All reabsorption processes are linked to the function of the basolateral Na+-K+-pump. The extremely high water permeability of the proximal tubule is essential for its nearly isosmotic volume reabsorption. The active reabsorption of solutes makes the fluid slightly dilute and the interstitial fluid slightly hypertonic. If inulin and PAH molecules are present their concentration in the fluid will rise (PAH also because of proximal secretion). The actively reabsorbed solutes have lower permeabilities (higher reflection coefficients) than NaCl. In the first half of the proximal tubule, Na+- is reabsorbed with carbonic acid and organicmolecules belonging to the reabsorption family. - The proximal and distal reabsorption ofbicarbonate is already described in Chapter 17. Fig. 25-9: Reabsorption of NaCl in the early and the late part of the proximal tubule. CA stands for carboanhydrase in the brush borders of the cell. The reabsorption family of substances (X) enters the tubule cells by specific symporter proteins coupled to the Na+ -reabsorption (1.in Fig. 25-9). This is secondary active transport showing saturation kinetics. Na+ -reabsorption is also coupled to H+ -secretion from the cell by the function of the Na+ -H+-antiporter protein (2. in Fig. 25-9). This H+ -secretion is linked to bicarbonate reabsorption in the upper part of the proximal tubules. The driving force for the Na+ -entry is the Na+ -K+-pump located in the basolateral membrane, which extrudes the Na+ to the intercellular space and the blood (3. in Fig. 25-9). Glucose is a typical example. The luminal membrane contains a sodium-glucose-cotransporter (SGLT 2). A genetic defect in this protein produces familial renal glucosuria – just as a genetic defect in a similar intestinal protein (SGLT 1) produces glucose-galactose malabsorption. - The passage of glucose across the basolateral membrane is by carrier-mediated (facilitated) diffusion. In the second half of the proximal tubule, Na+ is reabsorbed together with Cl- across the cell membrane or through paracellular routes (Fig. 25-9, below). In this segment the tubular fluid contains a high concentration of Cl- and a minimum of organic molecules. Na+ crosses the luminal membrane by the operation of Na+-H+-antiporters and Cl- -anion antiporters. In the tubular lumen the secreted H+ and anion form a H+-anion complex. The accumulation of a lipid-soluble H+-anion-complex establishes a concentration gradient that allows H+-anion-complex recycling (Fig. 25-9). Transfer of the Cl- -ion from the tubular fluid to the blood causes the tubular fluid to become positively charged relative to the blood. 4d. Reabsorption in the thick ascending limb The Na+-K+-pump maintains a low intracellular Na+ , which drives the simultaneous, electroneutral reabsorption of 1 Na+, 1 K+, and 2 Cl- by the luminal Na+‑K+-2Cl--symporter. The Cl--channels are only located in the basolateral membrane, so accumulated Cl- reaches the ISF. The K+-channels are located in all membranes and K+ recirculates (Fig. 25-10). Paracellular reabsorption of positive ions by diffusion is augmented by the positive charge of the tubular fluid (Fig. 25-10). The secondary active reabsorption of Na+ (and Cl-) is the basis for the transepithelial single effect gradient at each transverse level of the thick ascending limb (see later). Fig. 25-10: Reabsorption of NaCl in the thick ascending limb of the Henle loop. There is a luminal Na+ K+-2Cl--symporter and a basolateral Na+ K+-pump. This mechanism is essential for development of medullary hypertonicity by NaCl and thus for counter current mutiplication (see later). The electrochemical energy for the function of the basolateral Na+ K+-pump is provided by its Na+-K+-ATPase. The pump throws Na+ into the peritubular fluid. The K+ and Cl- ions leak out passively. The thick ascending limb is impermeable to water in the absence of ADH, and reabsorbs Na+ actively. Loop diuretics, which abolish the entire osmolar gradient in the outer renal medulla, inhibit the luminal Na+ K+-2Cl--symporter of the thick ascending limb. 4e. Reabsorption in the distal tubule and collecting duct The distal tubule is divided into an early and a late segment, since the early segment reabsorbs NaCl and is impermeable to water (as the thick ascending limb), whereas the late segment functions more like the collecting duct. In the early segment, the NaCl transfer is mediated by a NaCl-symporter (Fig. 25-11). Na+ leaves the cell through the basolateral Na+‑K+-pump, and Cl- leaves the cell by diffusion across the basolateral Cl--channels. Only a small fraction of the glomerular filtrate reaches the distal tubules. Thiazide diuretics inhibit the NaCl-symporter.Fig. 25-11: Cellular transport processes in the distal tubule and collecting duct. The late segment is composed of two cell types just as the collecting ducts. The light principal cells reabsorb Na+ and secrete K+. The Na+-K+-pump in the basolateral membrane draws Na+ out into the ISF and K+ into the principal cells (Fig. 25-11). These cells have special ion channels in the luminal membrane, which is permeable to Na+, but also to K+. The Na+-uptake depolarises the luminal membrane (-70 mV) and makes the lumen electronegative (-12 mV) compared to the interstitial fluid (reference potential zero). K+ rapidly diffuses into the tubular fluid. This secretion of K+ into the tubular fluid from the principal cell is thus linked to the Na+-reabsorption. The amount of Na+ reabsorbed in the distal tubule system is much less than in the proximal, but it can be increased by the adrenocortical hormone, aldosterone. Aldosterone is a mineralocorticoid, which promotes the reabsorption of Na+ (and thus Cl-) and the secretion of K+ (and H+) in principal cells. Aldosterone enters the cell from the blood and binds to an intracellular receptor to form a complex. The complex increases the formation of membrane proteins including the Na+-K+-pump and the luminal Na+-channels. This is the essential control mechanism for [K+] in the ECV. Secretion mainly occurs when the [K+] in the ECV is higher than normal. Aldosterone also promotes the reabsorption of Na+ (and thus Cl-) and the secretion of K+ (and H+) in the collecting ducts of sweat and salivary glands just as in the principal cells of the distal tubules of the kidney. Aldosterone-antagonists inhibit all aldosterone effects. The dark intercalated cells secrete H+ across the luminal membrane and reabsorb K+. Intercalated cells are mitochondrial-rich and most active in persons with a low K+-pool. The H+ -secretion by the H+-pump is precisely determined by the [H+] in the ECV.The collecting duct contains principal and intercalated cells just as the late distal segment, but the intercalated cell disappears in the inner medullary collecting ducts. The luminal membrane of the principal cells in the collecting ducts can be regulated from nearly water-impermeable (in the absence of antidiuretic hormone, ADH) to water-permeable (in the presence of ADH). The hormone increases the water-permeability by insertion of water-channels called aquaporin 2. The water-channels are stored in cytoplasmic vesicles that fuse with the luminal membrane. The basolateral membrane of the principal cell contains other aquaporins and they remain water-permeable even in the absence of ADH. Mutations in the genes for these channel proteins cause nephrogenic diabetes insipidus. 5. Tubular secretion and the PAH family Substances secreted like PAH constitute the secretion or PAH family. The filtration flux (Jfiltr) as usual increases in direct proportion to the rise in Cp (Fig. 25-12). Dividing the excretion flux for PAH with Cp provides us with the PAH clearance. The clearance is the slope of the excretion flux curve (Fig. 25-12). The secretion flux approaches a maximum (Tmax). Most of the PAH molecules are free, but 10-20% are bound to plasma proteins.Fig. 25-12: Renal PAH net rates (fluxes or J) as a function of plasma concentration, Cp. Organic acids and bases secreted in the proximal tubules include endogenous substances and drugs. The endogenous substances include adrenaline, bile salts, cAMP, creatinine, dopamine, hippurates, noradrenaline, organic acids and bases, oxalate, prostaglandins, steroids and urate. The drugs comprise acetazolamine, amiloride, atropine, bumetanide, chlorothiazide, cimetidine, diodrast, furosemide, hydrochlorothiazide, morphine, nitrofurantoin, para-aminohippuric acid (PAH), penicillin, phenol red, probenecid, sulphonamides, and acetylsalicylic acid. The secretion is often competitive. All these substances have varying but high affinity to an organic acid-base secretory system in the proximal tubule cells showing saturation kinetics with a Tmax. The organic cation secretion is analogous to the anion secretion.5a. Tubular handling of PAH Tmax is the maximum secretion rate for PAH in the tubules (Fig. 25-13). Normally, the Tmax is 0.40 mmol per min (80 mg/min) for PAH. At low PAH concentrations in the plasma (Fig. 25-13), the slope of the excretion rate curve is high (the clearance for PAH is high). Here the PAH clearance is an acceptable estimate of the minimal renal plasma flow (see effective RPF later), because the blood is almost cleared by one transit. The secretion flux is maximal, when the plasma-[PAH] is high enough to achieve saturation. The weak organic acids and bases mentioned above are similarly secreted into the proximal tubule, and have secretory Tmax -values just like PAH (Fig. 25-14). In humans of average size (with an average body surface area of 1.7 m2), the Tmax for diodrast and phenol red average 57 and 36 mg/min, respectively. 5b. Tubular handling of urate The active reabsorption of urate ions is accomplished in the proximal tubules by an electroneutral Na+-cotransport. The tubular reabsorptive capacity is normally far greater than the amount delivered in the glomerular filtrate. Above a critical concentration in the ECV of about 0.42 mM, the urate precipitates in the form of uric acid crystals, provided the environment is acid. Precipitation in the joints is termed gout (arthritis urica), often affecting several joints. Urate ions are accumulated in the ECV of gout patients, and often also in patients with uraemia. High doses of probenecid compete with urate for the proximal reabsorption mechanism. Use of this drug to patients with acute gout increases the excretion of urate in the urine. The active secretion of urate ions occurs from the blood plasma to the tubular fluid by the organic acid-base secretory system, which has a low capacity for urate. Thus, the renal tubules have a capacity of both actively reabsorbing urate ions and actively secreting them. 5c. Tubular handling of creatinine Essentially all creatinine in the glomerular filtrate passes on and is excreted in the urine. The molecule is larger than that of urea, and none of it is reabsorbed. Contrary, creatinine is secreted into the proximal tubules, so that the creatinine concentration in the urine increases more than 100-fold.5d. The secretion mechanism The molecules of the secretion family leave the blood plasma of the peritubular capillaries and binds to basolateral receptors with symporters on the tubule cell (Fig. 25-13). These channels are driven by energy from the basolateral Na+-K+-pump transporting the molecules against their chemical gradient across the basolateral membrane. Inside the cell the molecules accumulate until they can diffuse towards the luminal membrane. Here, an antiporter transfers the ions into the tubular fluid. All these molecules compete for transport, so intake of the drug probenecid can reduce the penicillin secretion loss.Fig. 25-13: Secretion of organic anions across the proximal tubules The luminal membrane contains specific receptor proteins for nutritive mono- and di-carboxylates. These receptor functions are also coupled to Na+ -transfer.6. Water and solute shunting by vasa recta The normal perfusion of the renal medulla is typically 5-10% of RBF. This bloodflow is larger than the fluid flow through the loop of Henle. Both the vasa recta and the closely located loops of Henle (from juxtamedullary nephrons) consists of two parallel limbs with counter-current fluid flow in the medulla. Vasa recta are designed as a counter current bloodflow and act as water-solute shunts that protect the medullary hyperosmotic gradient. The endothelial lining of vasa recta is highly permeable for small molecules (water, urea, NaCl, oxygen and carbon dioxide). Vasa recta also serve as a nutritive source to the medulla. Vasa recta receive blood from the efferent arterioles and consequently have an elevated colloid osmotic pressure and reduced hydrostatic pressure (Fig. 25-14). The net force in these vascular loops favours net fluid reabsorption. Let us consider the situation with a hyperosmotic medullary gradient and ADH present, so a concentrated urine is produced. The blood in the descending limb of vasa recta is first passed on in the direction of increasing medullary osmolarity. Accordingly, this blood must gradually supply water to the hyperosmolar, interstitial fluid by passive osmosis, and passively reabsorb solutes (NaCl and urea) by diffusion. Hereby, the interstitium is temporarily diluted and the blood is concentrated. In the ascending portion the blood passes regions with falling osmolarity, and the blood gradually absorbs water osmotically and delivers solutes to the interstitium by diffusion. The flow in the ascending vasa recta is larger than in the descending limb, because water from the Henle loop is also reabsorbed. Fig. 25-14: A: Passive counter-current exchange occurs in vasa recta, with diffusion of solutes along black arrows. Passive osmotic flux of water from the blood to the hyperosmolar interstitium occurs along stippled, blue arrows. – B: The active counter-current multiplier in the thick ascending limb with a single effect at each horizontal level. The gross effect of the passive counter-current exchange in the vasa recta is that of a water shunt passing the medullary tissue, whereas solutes recycle and thus are maintained in medulla. Water is shunted from limb to limb without disturbing the inner medulla. The passive counter-current exchange and low bloodflow through the vasa recta curtail the medullary hyperosmotic gradient (Fig. 25-14). The meagreness of the medullary blood flow, reduced by ADH, contribute to the maintenance of the medullary hyperosmotic gradient, but reduce the nutritive supply to the inner medulla. 7. Concentration or dilution of urine The thin ascending limb of Henle is impermeable for water, but highly permeable for NaCl and less so for urea. The thick ascending limb is also impermeable for water and also for urea. The water permeability of the cortical and medullary collecting ducts increase with increasing concentrations of antidiuretic hormone (ADH) in the peritubular blood. Concentration of urine. Initially, the osmolarity of the tubular fluid, the vasa recta blood, and the interstitial fluid is 300 mOsmol * l-1. The ascending limb of the Henle loop is impermeable to water and actively transports NaCl from the preurine into the surrounding interstitium. Thus solute and fluid is separated and the tubular fluid becomes diluted. At each horizontal level of the thick ascending limb, a hyperosmotic gradient (a single effect) of typically 200 mOsmol * l-1 is established (Fig. 25-14B). Energy is necessary to establish the hyperosmotic gradient. The energy is from Skou´s basolateral Na+-K+-pump, working in conjunction with the Na+-K+-2Cl—symporter of the thick ascending limb (Fig. 25-10). The total osmolarity in the inner medullary interstitial tissue can be as high as 1400 mOsmol per l, when the urine is maximally concentrated. The renal cortex fluid is isotonic with the plasma. When the isotonic fluid from the proximal tubules passes down through the hypertonic medulla in the descending thin limb of the Henle loop, water moves out into the medullary interstitium by osmosis, making the tubular fluid concentrated. This is because the epithelial cells of the thin descending limb are highly permeable to water but less so to solutes (NaCl and urea). Water is reabsorbed and returned to the body via vasa recta and the renal veins. At the bend of the loop the fluid has an osmolarity equal to that of the surrounding medullary interstitial fluid. However, the tubular fluid has a greater concentration of NaCl and a smaller concentration of urea than the surroundings. In contrast to the thin and thick ascending limb, most cell membranes including those of the proximal tubules and the thin descending limb of the Henle loop, are water-permeable under all circumstances. This is because these cell membranes contain water-channel proteins called aquaporins. As new fluid enters the descending limb of the Henle loop, the hyperosmotic fluid in the bottom of the loop is pushed into the ascending limb, where NaCl is separated from water. The osmolarity of the isosmotic tubular fluid running into the thin descending loop of the outer medulla is 300 mOsmol*l-1 and the output to the distal tubule is 100 mOsmol*l-1 (Fig. 25-14, B). At the bottom of the Henle loop the osmolarity can increase to at least 1300 mOsmol*l-1. In a steady state with continuous fluid flow the total osmotic gradient along the entire system is thus (1300 - 100) = 1200. The gradient along the entire system is 6 multiples of the 200 mOsmol*l-1 single effect gradient. The thick ascending limb is a counter-current multiplier with a high multiplication capacity. The NaCl is reabsorbed repeatedly in the thick ascending limb of the Henle loop. The passive counter-current exchange in the vasa recta and the active counter-current NaCl reabsorption in the thick ascending limb combine into a solute-water separator, when ADH is present. Another component in the maintenance of the medullary hyperosmotic gradient is addition of urea to the tubular fluid in the thin segment of the Henle loop. Urea is then trapped in the lumen, because all nephron segments, from the thick ascending limb through the outer medullary collecting duct, are impermeable to urea. As the tubular fluid flows through the distal tubules, cortical collecting ducts and outer medullary collecting ducts, its urea concentration rises progressively, because these segments are essentially urea-impermeable whether or not ADH is present. In the presence of ADH, water is reabsorbed but urea is not and the osmolarity of the fluid increases. The maximal osmolarity in the cortical collecting duct is up to 300 mOsmol*l-1, which is equal to the surrounding interstitial fluid. The distal fluid contains much urea and less NaCl. In reverse, the inner medullary collecting duct cells have urea-transporters that are ADH-sensitive. Thus large amounts of urea are reabsorbed at low urine flows, and the inner medullary interstitial fluid is loaded with urea that diffuses back to the tubular fluid through the thin descending and ascending limb in this urea recycling process. Urea covers 700 and NaCl also 700 mOsmol*l-1 out of the total 1400. Without passive urea recycling, the medullary interstitial osmolarity contributed by NaCl would have to double and thus the energy demand. Without the medullary hypertonic gradient we would be unable to produce concentrated urine when water depleted. A high osmolarity in the medullary interstitium enhances passive water reabsorption when ADH is present. ADH increases the concentration of solutes in the collecting ducts, and reduces the loss of water. A hyperosmotic concentration – moving from 300 up to 1400 mOsmol*l-1 in the inner medulla - has established a large concentration gradient between the tubular and the interstitial fluid. In man, the maximal urine osmolarity – when ADH is high - is 1400 mOsmol*l-1, which in a daily urine volume of 500 ml corresponds to a daily solute loss of up to 700 mOsmol. The small urine volume contains high concentrations of urea and nonreabsorbed or secreted solutes.Dilution of urine (large urine flow) In the absence of ADH, the distal tubules, cortical collecting ducts and outer medullary collecting ducts are impermeable to water. The osmolarity of the passing tubular fluid is reduced (towards 100 mOsmol*l-1) when we need a diluted urine. The medullary collecting duct reabsorbs NaCl (actively) and is slightly permeable to water and urea in the absence of ADH. The final urine – with small concentrations of NaCl and urea - has an osmolarity of 50-150 mOsmol*l-1, with a volume of up to 10% of the daily GFR. When ADH is absent, the fluid leaving the distal tubules remains hypotonic. Large amounts of hypotonic urine would then flow into the renal pelvis (with an osmolarity down towards 50 mOsmol*l-1). A daily solute loss of 700 mOsmol, under these conditions, implies a daily water loss of at least 14 l. The Fick's principle (mass balance principle) is used to measure the renal plasma clearance at low plasma [PAH] , since at low concentrations - the blood is almost cleared by one transit. Thus, the renal plasma clearance for PAH is almost equal to the renal plasma flow RPF in Eq. 25-5. The law of mass balance states that the infusion rate of PAH is equal to its excretion rate at steady state. Only one passage through the kidneys effectively eliminates PAH from the venous blood plasma at low [PAH]. A methodological short cut is to measure the [PAH] in the medial cubital vein only, instead of the true arterial [PAH] by arterial catheterisation. PAH clearance is an acceptable approximation called the effective renal plasma flow (ERPF). In a healthy, resting person the ERPF is 600-700 ml of plasma per min and lower than the RPF. The ERPF principle avoids complex invasive procedures such as catheterisations. The Tmax for PAH is also a valuable measure of the secreting tubular mass, because the proximal tubule cells are saturated with PAH at high plasma-[PAH]. The RBF falls drastically, when the mean arterial pressure is below 9.3 kPa (70 mmHg). The medullary bloodflow is always small in both absolute and relative terms. Any severe RBF reduction as in shock, easily leads to ischaemic damage of the medullary tissues resulting in papillary necrosis and ultimately to failure of renal function. During such pathophysiological conditions, prostaglandins (PGE2 and PGI2) are secreted from the mesangial and endothelial cells due to sympathetic stimulation. These prostaglandins dilatate the afferent and efferent glomerular arterioles and dampen the renal ischaemia caused by sympatho-adrenergic vasoconstriction. Both RBF and GFR show autoregulation following acute changes in the perfusion pressure within the physiological pressure range (Fig. 25-15). The renal autoregulation is mediated by myogenic feedback and by the macula densa-tubulo-glomerular feedback mechanism. Myogenic feedback is an intrinsic property of the smooth muscle cells of the afferent and efferent arterioles. The myogenic response allows preglomerular arterioles to sense changes in vessel wall tension (T) and respond with appropriate adjustments in arteriolar tone. Stretching of the cells by a rise in arterial transmural pressure (DP) elicits smooth muscle contraction in interlobular arteries and afferent arterioles (Fig. 25-15). During sleep the mean arterial pressure decreases 1-2 kPa, which would lower Pgc and GFR without autoregulation. Autoregulation with maintained RBF and GFR means that also the filtered load and the sodium excretion is maintained during sleep and variations in daily activities. The macula densa- TGF mechanism is described below. When the renal perfusion pressure rises, the cortical bloodflow is effectively autoregulated. However, during certain circumstances the papillary bloodflow may increase due to release of NO, prostaglandins, kinins or other factors. The increased medullary bloodflow increases the interstitial hydrostatic pressure and thus the resistance towards Na+-reabsorption, whereby the Na+-excretion increases. Sympathetic vasoconstriction reduces the renal perfusion pressure and thus the resting RBF. Increased renal sympathetic tone releases renin and enhances Na+-reabsorption in the proximal and distal tubules via nerve fibres. At maximum exercise RBF falls to half the resting level. - RBF also drops during emotional stress and during haemorrhage. Fig. 25-15: Pressure-flow relations in the kidney. The RBF curve shows autoregulation, and GFR follows the bloodflow. Noradrenaline/dopamine from adrenergic fibres and circulating adrenaline from the adrenal medulla, constrict the afferent and efferent glomerular arterioles, when the hormones are bound to a1-adrenergic receptors. This constriction decreases both RBF and GFR. Sympathetic stimulation releases renin from the granular JG-cells of the arterioles via b1-adrenergic receptors. Activation of the adrenergic fibres enhanches the Na+-reabsorption along the whole nephron. The normal 300-g's of kidney tissue receive a total bloodflow (RBF) of 1200 ml per min, which is 20-25% of the cardiac output at rest. Thus, on an average, RBF is 400 ml of blood per min and per 100-g kidney tissue. These units are actually called Flow Units (FU) or perfusion coefficients. The renal blood flow per weight unit is higher than any other major organ in the body. The renal cortex receives 90% of the total RBF, and only 5-10% reaches the outer medulla. The blood supply is at a minimum in the inner medulla, and the oxygen tensions falls off sharply in the papillary tissue. The medullary bloodflow can be reduced towards 1% by vasopressin. The counter current exchange of oxygen in vasa recta is a disadvantage to the renal papillae because their cells are last fed with oxygen by the blood. The inner cells meet their energy requirements primarily by anaerobic breakdown of glucose by glycolysis. The amount of energy obtained here is only 1/10 of the oxidative breakdown of 1 mol of glucose (2 888 kJ free energy). The cortical bloodflow is much larger than the medullary bloodflow. Here, 1/5 of the whole plasma stream passes the glomerular barrier by ultrafiltration and becomes preurine. Fortunately, we obtain the greater part of the energy required for cortical tubular transport by oxidative metabolism. 9. Macula densa-tubulo-glomerular feed-back (TGF) The macula densa-TGF mechanism responds to disturbances in distal tubular fluid flow passing the macula densa. The JG-apparatus includes 1) the renin-producing granular cells of the afferent and efferent arterioles, 2) the macula densa of the thick ascending limb, and 3) the extraglomerular mesangial cells connecting the afferent and the efferent arteriole (Fig. 25-16). Renin is described in paragraph 6 of Chapter 24. Fig. 25-16: The juxtaglomerular apparatus with renin secretion. Regulation of renal sodium excretion is described in paragraph 9 of Chapter 24. The TGF mechanism thus includes the renin-angiotensin II-aldosterone cascade (Fig. 24-5). Prostaglandins, adenosine and NO can modulate the response. These renin responses are part of the autoregulation to maintain RBF and GFR normal.Non-ionic diffusion is a passive tubular reabsorption of weak organic acids and bases, which are lipid-soluble in the undissociated or non-ionised state. In this state these compounds penetrate the lipid membrane of the tubule cell by diffusion. The tubule cells, however, are practically impermeable to the dissociated form of these compounds. Therefore, the ionic form of the weak acid or base is fixed in the tubular fluid and favoured for urinary excretion. A weak organic acid is mainly undissociated at low urinary pH, whereas an organic base is more dissociated. In acid urine the reabsorption rate of weak organic acids is increased, whereas the reabsorption rate of weak organic bases is reduced. In alkaline urine the opposite situation prevails. Examples of weak acids showing this phenomenon are phenobarbital and procain (both with pK just below 7), NH4+, acetylsalicylic acid, and many other therapeutics. Weak bases are the doping substance, amphetamine, and many therapeutics. In rare cases of poisoning with weak bases, the patients are treated with infusions of ammonium chloride solutions or amino acid-HCl solutions, which acidifies the urine (see Chapter 17). In cases of poisoning with weak acids, some patients receive infusions of bicarbonate solutions, whereby alkalisation of the urine is instituted. 11. Tests for proximal and distal tubular function Several proximal tests are available. 1. About 30 g of plasma albumin passes through the glomerular barrier each day. Fortunately, most of this albumin is absorbed through the brush border of the proximal tubules by pinocytosis. Inside the cell the protein molecule is digested into amino acids, which are then absorbed by facilitated diffusion through the basolateral membrane. Proteins derived from proximal tubule cells, such as ß2-microglobulin, are reabsorbed by the proximal tubules. If this protein is demonstrated by urine electrophoresis, a proximal reabsorption defect is present. This is also the case, when generalized aminoaciduria is present. 2. Glucosuria in the absence of hyperglycaemia indicates a proximal reabsorption defect of glucose, since all glucose is reabsorbed before the fluid reaches the end of the proximal tubules in the normal state. 3. The lithium clearance. The lithium ion, Li+, is filtered freely across the glomerular barrier, and its concentration in the ultrafiltrate is equal to that in plasma water. Lithium carbonate is used in the treatment of manic phases (catecholamine over-reaction) of manic depressive psychosis. A plasma concentration of 0.5-1 mM provides enough Li+ to block membrane receptors on the neurons involved for catecholamine binding. Fig. 25-17: Lithium clearance used as a measure of the proximal reabsorption capacity in the nephron. Li+ is reabsorbed isosmotically in the proximal tubules together with water and Na+ (Fig. 25-17). The amount of Li+ that leaves the proximal tubules (pars recta) is equal to its excretion rate in the final urine. This is because there is practically no reabsorption or secretion of Li+ distal to this location. Accordingly, a large lithium clearance depicts a low proximal lithium reabsorption, and thus a poor proximal tubular function at a given GFR. Normally, the passage fraction of Li+ is 0.25-0.3 at the end of the proximal tubules and almost the same fraction passes into the urine. 4 Hypokalaemia combined with normal or increased renal K+ -excretion suggests a defective proximal K+ -reabsorption (see Chapter 24 or Table 25-1). 5 Secretion across the proximal tubules (PAH clearance). Tests of distal tubular function:
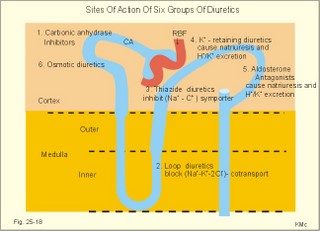
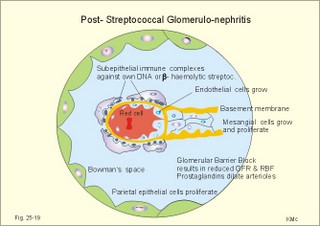
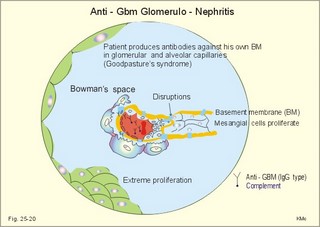
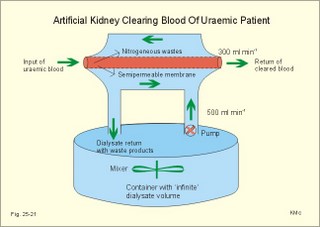
12. Stix testing with dipstics Routine stix testing for blood, glucose, protein etc. is necessary for the clinical evaluation of renal patients. Reagent strips for red blood cells are extremely sensitive. Even a trivial bleeding from a small capillary results in a positive answer indicating the presence of a few red cells. In such cases microscopy is necessary. Microscopy of fresh urine reveals red cells in cases of bleeding from the urinary tract, and red-cell casts in cases of kidney bleeding as in glomerulonephritis. Since the concentration threshold in urine for most reagent strips is 150 mg albumin per litre (l), there is no reaction to the normal albumin concentration of 20 mg l-1. Even 50-100 mg of protein is often excreted daily due to the upright posture and exercise. An early sign of diabetic glomerular leakage or nephropathy is microalbuminuria, which is defined as an albumin concentration of 50-150 mg per l of urine, and measured by radioimmunoassay (RIA). Some laboratories measure the Tamm-Horsefall glycoprotein, which is secreted from the cells of the thick ascending limb of Henle, and thus a normal constituent of urine. Bacteria in the urine produce nitrite from the urinary nitrate, and dipsticks easily demonstrate the nitrite. Urinary tract infection also results in white blood cells in the urine, and more than 10 cells per µl are abnormal. Diuretics are therapeutic agents that increase the production of urine. Diuretics are employed to enhance the excretion of salt and water in cases of cardiac oedema or arterial hypertension. The so-called natriuretics inhibit tubular Na+-reabsorption, but since the secretion of K+ and H+ is also increased, the patient must have compensatory treatment. The sites of action for different groups of diuretics are shown in Fig. 25-18. 13 a. Carboanhydrase inhibitors (eg, acetazolamide) act on the carboanhydrase (CA) in the brush borders and inside the cells of the proximal tubules. Inhibition of the metallo-enzyme reduces the conversion of filtered bicarbonate to carbon dioxide. As a result, there is a high concentration of bicarbonate and sodium in the tubular fluid of the proximal tubules. Up to half of the bicarbonate normally reabsorbed is eliminated in the urine causing a high urine flow and a metabolic acidosis. Thus, these inhibitors are diuretics. They are mainly used in the treatment of open-angle glaucoma (ie, an intraocular pressure above 22 mmHg). Acetazolamide promotes the outflow of the aqueous humour and probably diminishes its isosmotic secretion.Fig. 25-18: Sites of action on the nephron of different groups of diuretics 13 b. Loop diuretics (bumetanide and furosemide) inhibit primarily the reabsorption of NaCl in the thick ascending limb of Henle by blocking the luminal Na+-K+-2Cl--symporter. The reabsorption of NaCl, K+ and divalent cations is reduced, and also the medullary hypertonicity is decreased. Hereby, the distal system receives a much higher rate of NaCl, water in isotonic fluid, and K+. The overall result is an increased excretion of NaCl, water, K+ and divalent cations. The patient’s plasma- [K+] should be checked regularly. 13 c. Thiazide diuretics (bendroflurazide, hydrochlorothiazide) act on the early part of the distal tubule by inhibiting the (Na+- Cl-)-symporter. They increase K+ excretion by increased tubular flow rate. Thiazide and many other diuretics are secreted in the proximal tubules. This secretion inhibits the secretion of uric acid, so thiazide is contraindicated by gout. 13 d. Potassium-sparing diuretics (eg, amiloride) inhibit Na+-reabsorption by inhibition of sensitive Na+-channels in the principal cells of the distal tubules and collecting ducts. Hereby, they reduce the negative charge in the lumen and thus the K+-secretion. Amiloride causes natriuresis and reduces urinary H+- and K+-excretion 13 e. Aldosterone-antagonists (eg, spironolactone) compete with aldosterone for receptor sites on principal cells. As aldosterone promotes Na+-reabsorption and H+/ K+ -secretion, aldosterone-antagonists cause a natriuresis and reduce urinary H+ - and K+ -excretion. Aldosterone-antagonists are weak potassium-sparing diuretics, mainly used to reduce K+ -excretion caused by thiazide or loop diuretics. 13 f. Angiotensin-converting-enzyme (ACE)-inhibitors (captopril, enapril and lisinopril) reversibly inhibit the production of angiotensin II, reduce systemic blood pressure, renal vascular resistance and K+ -secretion. ACE-inhibitors promote NaCl and water excretion. ACE-inhibitors increase RBF without much increase in GFR, because of a decrease in both afferent and efferent arteriolar resistance. The development of diabetic nephropathy can be markedly delayed by early reduction of blood pressure with ACE-inhibitors and by careful diabetic management. 13 g. Osmotically active diuretics are substances such as mannitol and dextrans. These substances retard the normal passive reabsorption of water in the proximal tubules. Osmotic therapy with mannitol is used in the treatment of cerebral oedema. Mannitol is a hexahydric alcohol related to mannose and an isomer of sorbitol. Mannitol passes freely through the glomerular barrier and has hardly any reabsorption in the renal tubules. Its presence in the tubular fluid increases flow according to the concentration of osmotically active particles, which inhibit reabsorption of water. The high flow of tubular fluid means that the excretion of Na+ is great - despite the rather low Na+ concentration. Mannitol may help to flush out tubular debris in shock with acute renal failure, but the results are controversial. Dextrans (ie, polysaccharides) have a powerful osmotic and diuretic effect. - The larger, molecules (macrodex) are seldom used as volume expanders during shock because of allergic reactions. This paragraph deals with 1. Glomerulonephritis, 2. Renal insufficiency, 3. Acute tubular necrosis, 4. Diabetic nephropathy, 5. Nephrotic syndrome, 6. Urinary tract infection, 7. Tubulo-interstitial nephritis, 8. Gouty nephropathy, 9. Renal hypertension, 10. Urinary tract obstruction, and 11. Tumours of the kidney. The severity and cause of kidney disease is evaluated by measurement of the GFR. Glomerulonephritis is an immunologically mediated injury of the glomeruli of both kidneys. The majority of patients suffer from postinfectious glomerulonephritis or immune complex nephritis. This is a disorder, where circulating antigen-antibody complexes are deposited in the glomeruli or free antigen is bound to antibodies trapped in the capillary network. Typically, the antigen is derived from Lancefield group Aß- haemolytic streptococci, but also other bacteria, viruses, parasites (malaria), and drugs may be the origin. A few patients produce antibodies against their own antigens (eg, host DNA in systemic lupus erythematosus, malignant tumour antigen, or anti-glomerular basement antibody, anti-GBM). The inflammation is an abnormal immune reaction often caused by repeated streptococcal tonsillitis. An insoluble antigen-antibody complex precipitates in the basement membrane of the glomerular capillaries. The cells of the glomeruli proliferate, and disease will of course reduce GFR and to some extent, the RBF (measured as PAH clearance). Thus the infection depresses the glomerular filtration fraction (GFF = GFR/RPF). The acute postinfectious glomerulonephritis occurs typically in a child, who has suffered from streptococcal tonsillitis a few weeks before. Haematuria, proteinuria, and oliguria characterise acute nephritis with salt-water retention causing oedemas and hypertension. Pulmonary oedema and hypertensive encephalopathy with fits is life threatening. Uraemia is a clinical syndrome dominated by retention of non-protein nitrogen (eg, urea, uric acid, NH4+ creatinine and creatine). Uraemic patients generally exhibit hyperkalaemia (plasma- [K+] above 5.5 mM) and metabolic acidosis (pH below 7.35 and a negative base excess). This is due to the inadequate secretion of K+, NH4+ and H+. In complete renal shutdown, the patient dies within 1-2 weeks without dialysis. Dialysis is mandatory with severe uraemia. When serum creatinine rises above 0.7 mM, renal insufficiency is usually terminal (Fig. 25-4). Recording of blood pressure and fluid balance with weighing is important in order to prevent hypertension and pulmonary oedema to develop into a life-threatening condition. Fig. 25-19: Post-streptococcal glomerulonephritis. The parietal and visceral epithelial cells of the glomeruli grow and proliferate, just as the mesangial cells (Fig. 25-19). This proliferation and the damage of the basement membrane with accumulation of insoluble complexes all impair the glomerular barrier and reduce the glomerular filtration rate (GFR). Production of cytokines and autocoids enhance the inflammation. Capillary injuries with reduction of the lumen also reduce the renal bloodflow (RBF) to some extent (Fig. 25-19). Children with poststreptococcal glomerulonephritis are treated with a course of penicillin - often with an excellent prognosis. Glomerulonephritis as a part of systemic lupus erythematosus (SLE) is frequent in female lupus patients - in particular during pregnancy, where hypertension may precipitate glomerular injuries. Oestrogens accelerate progression of SLE, and there is a genetic predisposition. In SLE there is hyperactivity of the B-cell system, which may involve any organ, but typically affects the kidneys, joints, serosal membranes and the skin (Chapter 32). The B-cell system releases many antibodies to host antigens both in and outside the cell nuclei (single- and double-stranded DNA, RNA, plasma proteins, cell surface antigens, and nucleoproteins). Lymphocytotoxic antibodies are also liberated, which may explain the inhibition of the T-cell system. The most important autoantibodies are those against nuclear antigens. Accumulation of immune complexes with double-stranded DNA probably causes the glomerular lesions as well as vasculitis and synovitis. Fig. 25-20: Anti-GBM glomerulonephritis with anti-GBM of the IgG type. Complement is shown as a small circle. Anti-GBM glomerulonephritis is a seldom disorder, where the patient produces antibodies (IgG type) against his own basement membrane. The antibody is known as anti-GBM or anti-Glomerular Basement Membrane antibody. The antigen is localised both in the glomerular basement membrane and in the basement membrane of the alveolar capillaries. The histological picture is characterized by proliferation of both parietal epithelial cells, and mesangial cells (Fig. 25-20). The capillary basement membrane is disrupted, and there is red cells and fibrin in Bowmans space. The diagnosis is confirmed by identification of circulating anti-GBM (Y-shape in Fig. 25-20). Glomerulonephritis with pulmonary haemorrhage is termed Goodpastures syndrome. The recurrent haemoptyses can be life threatening. Renal insufficiency is a clinical condition, where the glomerular filtration rate is inadequate to clear the blood of nitrogenous substances classified as non-protein nitrogen (urea, uric acid, creatinine, and creatine). The retention of nonprotein nitrogen in the plasma water is called azotemia, and the clinical syndrome is called uraemia. The number of filtrating nephrons falls below 1/3 of normal, as determined by measurement of a GFR below 40 ml/min. Acute renal insufficiency accompanies extremely severe states of circulatory shock (prerenal cause). The prerenal causes are hypovolaemia with hypotension or impaired cardiac pump function or the combination. Also a large group of renal causes to failure occurs (Table 25-2). Finally, the postrenal causes are all types of urinary tract obstruction. Acute renal failure is a serious disorder, which leads to progressive uraemia and chronic renal insufficiency.
Two complications to chronic renal failure must be considered: 1. Renal osteodystrophy develops in patients with severe renal failure. The kidneys fail in producing sufficient 1,25-dihydroxy-cholecalciferol. This is active vitamin D or a potent steroid hormone. The active vitamin D metabolite stimulates the Ca2+-transport across the cell and mitochondrial membranes. Lack of active vitamin D has the following two effects: a. Poor gut absorption of dietary Ca2+, so that plasma [Ca2+] falls. b.The PTH release is stimulated, because the normal inhibitory effect of active vitamin D is lost. After some time a secondary hyperparathyroidism develops with increased resorption of calcium from bone and increased proximal tubular reabsorption of calcium in an attempt to correct the low serum calcium. The calcium release from bone results in osteomalacia and in osteoporosis. Osteomalacia or soft bones is the result of demineralisation of the osteoid matrix usually caused by insufficient active vitamin D. Osteoporosis or thin bones is characterized by a reduction in all components of the bones. 2. Normochromic, normocytic anaemia. When normal kidneys are perfused with hypoxaemic blood, the peritubular interstitial cells produce large amounts of the glycoprotein hormone, erythropoietin, with strong effect on erythrogenesis. Chronic renal failure leads to erythropoietin deficiency, and thus to anaemia, which is of the normochromic, normocytic type. The aim of haemodialysis is to eliminate nitrogenous wastes in patients with renal failure, and maintain normal electrolyte concentrations, serum glucose and normal ECV. In other words, the haemodialyzer or artificial kidney mimics the normal renal excretion of waste products (Fig. 25-21)Fig. 25-21: An artificial kidney (dialyser) with an area of 1 m2 and a membrane thickness of 10 µm. Blood from the patient is pumped through a container with series of semi-permeable membranes separating the blood from dialysate (Fig. 25-21). Dialysate is a mixture of purified water with salts, and glucose in a composition comparable to normal fasting plasma apart from proteins. Bicarbonate or acetate buffer is present at a concentration about 35 mM. Haemodialysis is performed with a bloodflow of 200-300 ml per min. The patient is often connected to the dialyzer by an arteriovenous shunt made by plastic cannulae between the radial artery and an adjacent vein. The arterial blood flows into the artificial kidney and after dialysis the blood is returned to the venous system (Fig. 25-21). Dialysate is pumped through the container at a rate of 500 ml each min. A plastic shunt connects the two cannulae on the forearm between dialysis sessions, and the large arterial bloodflow is sufficient to avoid coagulation in the plast shunt. Also dual-lumen venous catheters placed centrally are in use. If the sodium concentration of the dialysate is too high, the patient complains of thirst and the arterial pressure starts to rise. Low dialysate calcium may result eventually in secondary hyperparathyroidism, whereas a high dialysate calcium concentration causes hypercalcaemia. An adult patient with acute renal failure (so-called shock kidney) requires 4 -5 hours dialysis 3 times a week. Fit patients with chronic renal failure are offered renal transplantation. Rejection of the transplant is due to complement-fixing antibodies in the blood, or later caused by cellular or humoral immunity. Rejection years after the transplantation is frequently caused by ischaemic damages of the kidney. Donation of a kidney leaves the donor with one kidney only. Immediately after the removal, the GFR of the patient falls to half its original value, because half the functioning nephrons have been removed. Soon, most individuals will increase their GFR towards normal values by compensatory work hypertrophia of the remaining kidney. The hypertrophia-factor is not known. Each remaining nephron must filter and excrete more osmotically active particles than before. This disorder has haemodynamic or toxic causes. Cardiogenic and hypovolaemic shock cause acute renal failures just as renal vasoconstriction. Renal ischaemia leads to hypoxic damage, in particular damage of the renal medulla, which is especially susceptible to ischaemia, because of the normally relatively poor oxygenation. Ischaemic tubular damage also reduces the GFR further, because of reflex spasms of the afferent arterioles, and due to tubular blockage with accumulation of filtrate in the early part of the proximal tubules, and hypoxic damage of the proximal tubular reabsorption capacity. Loss of appetite and energy, nausea and vomiting, nocturia and polyuria characterise the condition. Only when the GFR is severely depressed there is oliguria. Even a GFR of only 1 ml each min, as a contrast to the normal 125 ml per min, may result in a daily urine flow of 1440 ml (1*1440 min daily), if there is a total loss of tubular reabsorption and no luminal obstruction. This urine flow is normal, but unfortunately based on an almost total loss of glomerular and tubular function. Sufficient regeneration of the tubular epithelium allows clinical recovery. Sometimes also the renal cortex is necrotic, and following healing of the injuries, the result is scarring with glomerulosclerosis. This condition is also found following radiation nephritis. Diabetic nephropathy includes glomerulosclerosis, with thickening of the basement membrane and damage of the glomerular filter by disruption of the protein cross-linkages and glomerular hyperfiltration. Excess NO production reduces the afferent arteriolar resistance and increases the glomerular capillary pressure. The earliest evidence of glomerular damage may occur 5-15 years following diagnosis in the form of microalbuminuria. The patient later develops intermittent albuminuria followed by persistent albuminuria. Diabetic nephropathy includes hypertension, persistent albuminuria, and a decline in GFR. One third of all insulin-dependent diabetics develop nephropathy. The mortality rate is high. The metabolic disturbance in diabetics causes hypertension and leaky renal glomeruli, but the mechanism remains uncertain. Ascending infections result in interstitial lesions and diabetes typically show hypertrophy and hyalinization of afferent and efferent arterioles. Obstruction of the renal bloodflow (ischaemia) leads to hypoxic damage of the renal tissue. The tenuous bloodflow to the renal papillae via the vasa recta explains why renal papillary necrosis is frequent in diabetics. Treatment with ACE- inhibitors reduce urinary albumin excretion. Prophylactic therapy also postpones the development of diabetic nephropathy and hypertension with persistent microalbuminuria. The effectiveness of this treatment suggests that relative oversecretion of angiotensin may be involved in the pathogenesis of diabetic nephropathy. The nephrotic syndrome refers to a serious increase in the permeability of the glomerular barrier to albumin, resulting in a marked loss of albumin in the urine. The albuminuria (more than 3 g per day) causes hypoalbuminaemia and generalized oedema. The number and size of pores in the glomerular barrier increase due to disruption of protein-linkages. Negatively charged glycoproteins in the glomerular barrier repel negatively charged proteins. The amount of negatively charged glycoproteins is reduced in glomerular disease. Oedema is visible in the face - especially around the eyes.A serious but rare complication may develop when a large volume of fluid accumulates in the abdominal cavity as ascites. Urination (micturition) is controlled by the micturition reflex. Stretch or contraction of the smooth muscles in the bladder wall is sensed by mechanoreceptors and signalled via the pelvic nerve to the sacral spinal cord. Increased parasympathetic tone (via pelvic nerves and muscarinic receptors) cause sustained bladder contraction. Normally, contraction of the bladder muscles by micturition almost completely empties the bladder. Recurrent infections of the urinary tract are frequent among females. Faecal bacteria are transferred to the periurethral region, and finally to the bladder via the short female urethra. Bladder urine is normally sterile owing to bladder mucosal factors and other local defence mechanisms. Bacteria adhere to the bladder epithelium and multiplicate, when defence mechanisms function insufficiently. Prolonged bladder catheterisation predisposes to bladder infection, and even a few days can be critical. The diagnosis bladder infection is based on more than 100 000 bacteria per ml of clean-catch mid-stream urine. Quite a few patients with significant bacteriuria do not develop nitrite enough to be shown by dipstick tests. Typical symptoms are frequent micturition (polyuria), painful voiding (dysuria), suprapubic pain and smelly urine perhaps with haematuria. Echerichia coli and other coliform bacteria cause the majority of urinary tract infections; these infections are treated successfully with antibiotics (amoxyllin, trimethoprim etc) either as a single shot or for longer periods.7. Tubulo-Interstitial Nephritis Bacterial pyelonephritis typically causes interstitial inflammation of the kidneys, but the interstitial inflammation is more often caused by a hypersensitivity reaction to drugs (antibiotics, phenacetin and non-steroid anti-inflammatory drugs, NSAIDs). Pyelonephritis begins in the renal pelvis, and then progresses into the renal medullary tissue. The essential function of the medulla is to concentrate the urine during water depletion. Therefore, in patients with pyelonephritis, the ability to concentrate the urine is abolished/decreased (isosthenuria/hyposthenuria). The ability to dilute the urine deteriorates also. Thus, in isosthenuria the urine is always isotonic with the plasma. The patient with acute nephritis has fever, skin rashes and acute renal failure with eosinophiluria and eosinophilia. First of all the offending drug must be withdrawn, and the renal failure may require dialysis. Chronic tubulo-interstitial nephritis is caused by pyelonephritis, NSAIDs, diabetes mellitus, hyperuricaemia, irradiation damage etc. The major problem is that long lasting consumption of large amounts of analgesics leads to terminal renal failure. Nephrotoxic analgesics must be abandoned.The patient presents with uraemia, albuminuria, polyuria, haematuria, anaemia, and most often a history of analgesic abuse. Papillary necrosis can be present with papillary tissue passed in the urine or obstructing the ureter or urethra. In patients with tubular damage of the renal medulla, the ability to concentrate the urine is abolished together with the ability to dilute the urine. Thus, the urine is always isotonic with the plasma (isosthenuria). The result is polyuria and salt wasting. As the inflammation progresses to the cortex also the glomerular filtration deteriorates with accumulation of non-protein nitrogen in the plasma water (azotaemia), and the clinical syndrome uraemia. An isolated damage of the Na+ -reabsorption (salt-losing nephritis) is a condition in which the disease processes are mainly due to dysfunction in the renal medulla. There is a marked loss of Na+ in the urine and seriously low ECV and blood volume (hypovolaemia with threat of imminent shock). Thus the patient must have a high salt intake to prevent shock and keep alive. Acute hyperuraemic nephropathy occurs in patients, where the condition leads to rapid destruction of cell nuclei (at the start of treatment for malignant disorders or obesity). Large quantities of nucleoproteins are released, and the production of uric acid is increased. The urate concentration increases in the extracellular volume (ECV). Above a critical concentration of 420 mM, the urate precipitates in the form of uric acid crystals, provided the fluid is acid. This concentration threshold defines hyperuricaemia. Precipitation in the joints with pain is termed gout (arthritis urica), and precipitation of uric acid crystals also occurs in the tubules, the collecting ducts and the urinary tract. Normally, urate ions are actively reabsorbed in the proximal tubules by a Na+-cotransport. Urate ions can also be actively secreted from the blood to the tubular fluid. Allopurinol is prescribed during radiotherapy or cytotoxic therapy. Acute cases are also treated with allopurinol and forced alkaline diuresis. Uric acid stones are found in patients with hyperuricaemia, and in patients secreting sufficient urate without hyperuricaemia. Calcium stones may be formed around a nucleus of uric acid crystals. Bilateral renal disease such as chronic glomerulonephritis is a frequent cause of hypertension (Chapter 12), whereas unilateral renal disease, such as renal artery stenosis, is a fairly seldom cause of hypertension. Stenosis (narrowing of the lumen) of one renal artery leads to renal hypotension with excess renin production (see below) and systemic (secondary) hypertension. Exposure to fluid loss, reduced glomerular propulsion pressure, and increased sympathetic activity releases renin from the juxtaglomerular cells in the afferent glomerular arteriole, so the renin-angiotensin-aldosterone cascade is triggered (Fig. 24-5). Angiotensin II stimulates the aldosterone liberation from zona glomerulosa of the adrenal cortex, and thus stimulates Na+ -reabsorption and K+ -secretion in the distal tubules. The result is salt and water retention with increase in blood volume and blood pressure. Angiotensin II also constricts arterioles, with an especially strong effect on the efferent renal arteriole. This reduces the renal bloodflow further and also the proximal reabsorption. The development of hypertension in high renin states is mainly due to salt-retention and systemic vasoconstriction.Stenosis of one renal artery does not always lead to increased erythrogenesis. Stenosis of the renal artery implies a small renal bloodflow, a small glomerular filtration and a small NaCl-reabsorption with a related small oxygen consumption on the affected side. As long as the renal oxygenation is sufficient, the erythropoietin production is normal. Severe renal artery stenosis implies renal ischaemia and hypoxia, which is probably always consequential with complications. A hypoxic kidney has a low creatinine and PAH clearance. A long-term increase in sodium intake results in changes of the kidney function. Surprisingly, the changes are similar in hypertensive and normotensive humans! Most people increase their ECV and GFR without changing the absolute reabsorption rate of Na+ and water in the proximal tubules. Therefore, the rise in filtration rate of Na+ and water will reach the loop of Henle and the distal tubule. The arterial blood pressure and heart rate is unaffected by the amount of sodium in the diet. The plasma concentrations of active renin (Fig. 24-7), angiotensin II and aldosterone decrease with increasing Na+ intake, but atrial natriuretic factor (ANF) and cyclic GMP increase. Arginine vasopressin (ADH) in plasma does not change. The reason why this increase in NaCl load to the loop of Henle is not counterbalanced by the TGF-system is due to resetting of the TGF-mechanism, so a contraction is avoided in spite of the increased salt load.These homeostatic reactions are all appropriate physiological responses in both healthy and hypertensive humans. A rare cause of renal hypertension is due to Liddles syndrome. This is an autosomal dominant defect characterised by severe hypertension, hypokalaemia and metabolic alkalosis. The syndrome is similar to primary hyperaldosteronism, but the renin-aldosterone concentration in plasma is not increased. Liddles syndrome is caused by mutation of the gene for the amiloride-sensitive Na+-channel (Fig. 25-11), whereby the channel is wide open. The Na+-entry depolarises the membrane and favours secretion of K+ and H+.Obstruction of the urinary tract may occur at any location, and cause dilatation of the above structures. The obstruction is localised within the lumen (stone, sloughed papilla, or tumour), within the wall (neuromuscular dysfunction, stricture, congenital urethral valve, or pin hole meatus), or pressure from the outside obstruct the tract (eg, tumours, diverticulitis, aortic aneurysm, prostatic obstruction, retrocaval ureter). Stretching of the renal calyces as they collect urine promotes their pacemaker activity and initiate a peristaltic contraction along the smooth muscle syncytium of the urinary tract. Obstruction of the urinary tract for weeks may lead to irreversible damage of the renal function in particular when combined with infection. Obstruction of the upper urinary tract with backpressure damage of the kidney is especially dangerous. Kidney stone disease (nephrolithiasis) attacks only a few percent of the Western population at any time. Most stones in male patients are composed of calcium complexed with oxalate and phosphate, whereas magnesium ammonium phosphate/acetate stones are more common in females. Only a few percent of all renal stones are composed of uric acid crystals or cysteine (mainly in children). Calcium-containing and cysteine stones are radiopaque, whereas stones of pure uric acid are radiolucent. In the presence of infection with urea-splitting bacteria, urea is hydrolysed to form the strong base ammonium hydroxide: CO (NH2)2 + H2O è 2 NH3 + CO2 ; NH3 + H2O è NH4+ + OH-. Alkaline urine favours stone formation by crystallization in the supersaturated fluid. Magnesium ammonium phosphate stones are also termed mixed infection stones. Obstruction or spasm of the ureter causes reflex constriction around the stone with ureteric or renal colic pain. The pain is an excruciating flank pain, with radiation to the iliac fossa and the genitals. The wall of the ureter is innervated with sensory nerve fibres running in the pelvic nerves. Renal colic is considered to be one of the most severe pain experience known. Excretion urography and plain X-ray examination are important in the diagnosis of renal stone disease.Percutaneous nephrolithotomy, pyelolithotomy or ureterolithotomy can avoid many cutting operations. Also shock-wave disintegration is in use (lithotripsy). Nephrocalcinosis refers to diffuse renal calcification that is detectable on a plain abdominal X-ray. Patients with hypercalcaemia (eg, primary hyperparathyroidism, hypervitaminosis D, and sarcoidosis) or with hyperoxaluria precipitate calcium oxalate and calcium phosphate in the renal parenchyma. Patients with renal tubular acidosis fail to acidify their urine, which favour precipitation of calcium oxalate and phosphate.Abdominal radiography A plain X-ray can identify calcification at any site including the renal system. Intravenous pyelography An organic iodine-containing contrast substance is injected slowly. Serial X-rays are taken, while compression bands are applied to the abdomen in order to obstruct ureteral emptying. Hereby, the upper renal tract is distended by the excreted contrast medium. Following removal of the compression bands, the rate of excretion of contrast is studied with films before and after voiding.Benign and malignant tumours occur in the kidney. Benign renal fibroma, cortical adenomas or simple cysts seldom cause symptoms and signs. Those of no clinical importance are found incidentally at autopsy. Juxtaglomerular cell tumours are seldom. They produce large amounts of renin, which causes hypertension.Haemangiomas may bleed following trauma and cause fatal blood loss. Malignant renal tumours are nephroblastoma and renal cell carcinoma. Nephroblastoma (Wilms´ tumour) is the most frequent intraabdominal tumour in both girls and boys. It usually presents within the first three years of life. A large abdominal mass is found sometimes with signs of intestinal obstruction. The tumour grows rapidly and spread to the lungs. The diagnosis is confirmed with excretion urography, arteriography or scanning.Radiotherapy and chemotherapy, combined with nephrectomy have improved the long-term survival rate. Renal cell carcinoma (hypernephroma) accounts for more than 90% of all the malignant renal tumours in adults - in particular smokers. There is a strong association with a rare autosomal dominant inherited disease called Von Hippel-Lindau´ syndrome (haemangioblastomas in the cerebellum and the retina). The genetic locus is on chromosome 3p.The tumour arises from proximal tubular epithelium, and lies within the kidney, but the prognosis is worse, if the tumour penetrates the renal capsule. The tumour is often protruding and the neoplastic cells have an unusually clear cytoplasm. Renal cell carcinoma is a likely source of ectopic hormone production. Increased production of erythropoietin leads to erythrocytosis and polycythaemia. Release of a parathyroid-hormone-like substance leads to hyperparathyroidism and hypercalcaemia. Release of abnormal quantities of renin triggers the renin-angiotensin-aldosterone cascade and leads to systemic hypertension. Metastases to distant regions are frequently found in the lungs and in the bones (osteolytic metastases). Solitary tumours are treated by partial or total nephrectomy or with interferon. · The plasma clearance is defined as follows: Eq. 25-1: Clearance = (Cu ×V°u) /Cp [(mg/ml)×(ml/min)/(mg/ml)= ml/min]. Clearance can also be thought of as the volume of arterial plasma containing the same amount of substance as contained in the urine flow per minute. · Excretion fraction (EF). EF for a substance is the fraction of its glomerular filtration flux, which passes to and is excreted in the urine. EF = Jexcr/Jfiltr Since Jexcr = (Cu ×V°u) and Jfiltr =(GFR × Cfiltr) it follows that: Eq. 25-2: EF = (Cu ×V°u) /(GFR × Cfiltr) Cfiltr is the concentration of the substance in the ultrafiltrate. The excretion fraction for inulin is one (1). Substances with an EF above one are subject to net secretion. Substances with an EF below one are subject to net reabsorption. · Extraction fraction (E). E for a substance is the fraction extracted by glomerular filtration from the total substance delivery to the kidney via renal blood plasma.Eq. 25-3: E = Jfiltr/Jtotal = (Ca - Cvr)/Ca. Substances with an E of one are cleared totally from the plasma during their first passage of the kidneys. Inulin has an extraction fraction of 1/5. PAH has an extraction fraction of 0.9.· Inulin clearance. The flux of inulin filtered through the glomerular barrier per min is: (GFR × Cp/0.94). All inulin molecules remain in the preurine and is excreted in the final urine. Thus, the amount excreted is equal to the amount filtered: GFR × Cp/0,94 = (Cu ×V°u) mmol/min Eq. 25-4: GFR = ((Cu ×V°u) /Cp) × 0.94 = CLEARANCEinulin × 0.94. · The Fick's principle (mass balance principle) is used to measure the renal plasma clearance at low plasma [PAH], since at low concentrations the blood is almost cleared (90%) by one transit. Thus the renal plasma clearance is equal to the effective renal plasma flow (ERPF): Eq. 25-5: ERPF = Jexcr/Cp ; RPF = ERPF/EPAH · The law of mass balance states that the delivery of PAH to the kidney is equal to its excretion rate at steady state. The Effective Renal Blood Flow (ERBF) is calculated by the help of a total body haematocrit (normally 0.45). If ERPF is measured to be 600 ml plasma per min, we can calculate ERBF: 600/(1 - 0.45) = 1090 ml whole blood per min at rest. This is 20-25 % of cardiac output. The true RBF is 10% higher than the measured ERBF (ie, 1200 compared to 1090 ml whole blood). The following five statements have True/False options: A: The B-cell system releases antibodies to host antigens. B: The glomerular barrier facilitates the passage of negatively charged polyanionic macromolecules. C: Thiazide diuretics may have serious side effects such as hypercholesterolaemia, hyperglycaemia (eg, glucose intolerance), hyperuricaemia, hypokalaemia, and impotence.D: Loop diuretics inhibit the reabsorption of NaCl in the thick ascending limb of Henle – and proximal pars recta - by blocking the cotransport process in the luminal entry membrane. E: Aldosterone antagonists, such as spironolactone, act on the aldosterone receptors on the late distal tubule cell and inhibit the K+-excretion. A male office worker, 58 years of age, body weight 70 kg, suffers from insulin-dependent diabetes mellitus. The disorder is complicated with arterial hypertension, hypercholesterolaemia, albuminuria and open-angle glaucoma. The patient is in anti-hypertensive therapy with a ß-adrenergic antagonist. The open-angle glaucoma is treated with acetazolamide (a carboanhydrase-inhibitor used as a diuretic to reduce the intra-ocular pressure). Scanning of the kidneys show a normal picture with an estimated normal kidney weight of 300 g. During renal catheterisation, a renal arteriovenous oxygen content difference is measured to 15 ml per l of blood, and the renal bloodflow is 1.2 l (normal). – The first 3 questions necessitate pharmacological knowledge.
A female patient (weight 57-kg) of 23 years, with an inherited defect in renal tubular function, has a lowered tubular threshold for glucose reabsorption. The patient has a blood- [glucose] of 1000 mg per litre, and just above this level glucose appears in the urine (her appearance threshold). The diuresis is 1.5 ml per min, the plasma -[creatinine] is 0.09 mM, and the urine [creatinine] is 6 mM. The normal blood-glucose level is 5-6 mM. 1. Is the above blood -[glucose] normal? 2. Calculate the creatinine clearance? 3. Calculate the glucose reabsorption at this glucose level and compare it to the normal maximal capacity: 1.78 mmol min-1. 4. Is the appearance threshold defined above equal to the saturation threshold? A 14-year old girl has a history of previous upper respiratory tract infections, and is now treated for another sore throat (ie, tonsillitis and high fever) with ampicillin for 10 days. Two weeks later she returns to her general practitioner (GP) complaining of tender knee joints from playing handball. There is abdominal pain. The girl is obviously ill and has a higher blood pressure than normally (145/90 mmHg or 19.3/12.7 kPa). The tonsillitis is cured and there is no fever. The upper abdomen is tender. A freshly passes urine sample is examined with a combined quantitative stick test. There is found haematuria and albuminuria (300 mg l-1). 1. What is the cause of the arthritis? 2. What are the causes of the haematuria and albuminuria? 3. Does the GP admit the girl to a hospital? During her working hours a 24-year old nurse delivered an arterial sample for blood gas tensions. She had no symptoms or signs of disease, but doubted that an arterial sample could be taken without causing pain. The sample was taken from a radial artery with a fine needle following local anaesthesia and she experienced no pain. The arterial blood gas values were: CO2 partial pressure 24 mmHg, O2 partial pressure 102 mmHg, pHa 7.36, and Base Excess - 8 mM. The nurse had been starving for 24 hours. 1. What was the explanation of her acid-base disturbance? 2. What was the rational treatment? A young female (body weight 56 kg) with an inulin clearance of 125 ml of plasma per min is tested with para‑amino‑hippuric acid (PAH). The free fraction of PAH in the plasma is 0.80, and the rest binds to plasma proteins. Her urine is collected in a period and the excretion flux of PAH is measured to 100 mg each min. The average concentration of PAH in plasma from the renal arterial and venous blood is 0.2 and 0.02 g per l, respectively. The haematocrit is 43%. 1. Calculate the clearance for PAH. 2. Calculate the tubular secretion flux for PAH at the blood plasma concentration concerned. 3. Calculate the renal blood flow (RBF). The patient collects the urine in a second period, where the average concentration of PAH in plasma from the arterial blood is 1 g per l. The maximal tubular secretion rate for PAH is defined as Tmax for PAH and is 80 mg per min. 4. Calculate the excretion flux for PAH in the urine. 5. Calculate the new clearance for PAH. Try to solve the problems before looking up the answers · Creatinine clearance provides a fair clinical estimate of the renal filtration capacity. · The renal control of body fluid osmolality maintains the normal cell volume (ICV) by changes of renal water excretion. · Normally, we excrete 1500 (range: 1200-1800) ml of water and 2-5 g of Na+ (= 5-12 g NaCl) daily. · Renal excretion of waste products. Urea from amino acids is excreted with about 30 g or half a mol of urea per day. The daily renal excretion of uric acid, creatinine, hormone metabolites and haemoglobin derivatives matches their daily production. · The daily renal excretion of metabolic intermediates and foreign molecules (drugs, toxins, chemicals, and pesticides) is carefully matched to the intake or production. · Secretion of hormones: The kidney secretes erythropoietin, renin, kinins, prostaglandins and 1,25-dihydroxy-cholecalciferol. · Acute Tubular Necrosis has haemodynamic or toxic causes. Cardiogenic and hypovolaemic shock cause acute renal failures just as renal vasoconstriction. Renal ischaemia leads to hypoxic damage, in particular damage of the renal medulla. Ischaemic tubular damage also reduces the GFR further, because of reflex spasms of the afferent arterioles, and due to tubular blockage with accumulation of filtrate in the early part of the proximal tubules. · Bacterial pyelonephritis typically causes interstitial inflammation of the kidneys, but the interstitial inflammation is more often caused by a hypersensitivity reaction to drugs (antibiotics, phenacetin and non-steroid anti-inflammatory drugs, NSAIDs). · Diabetic nephropathy includes hypertension, albuminuria and low GFR with glomerulosclerosis (thickening of the basement membrane and damage of the glomerular filter by disruption of the protein cross-linkages). The earliest evidence may be microalbuminuria. The patient later develops intermittent albuminuria followed by persistent albuminuria. · Nephroblastoma (Wilms´ tumour) is the most frequent intraabdominal tumour in both girls and boys. A large abdominal mass is found sometimes with signs of intestinal obstruction. The tumour grows rapidly and spread to the lungs. The diagnosis is confirmed with excretion urography and arteriography. · Renal cell carcinoma (hypernephroma) accounts for more than 90% of all the malignant renal tumours in adults (smokers). There is a strong association with a rare autosomal dominant inherited disease called Von Hippel-Lindau syndrome (haemangioblastomas in the cerebellum and the retina). The genetic locus is on chromosome 3p. Nephron. Monthly journal published by the International Society of Neprology. S Karger AG, Allschwilerstrasse 10, PO Box CH-4009 Basel, Switzerland. Rehberg, P. Brandt. "Studies on kidney function: I. The rate of filtration and reabsorption in the human kidney." Biochem. J. 20: 447, 1926. Schafer, JA. Renal water and ion transport systems. Am. J. Physiol. 275 (Adv. Physiol. Educ. 20): S119-S131, 1998.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Click here to introduce your comments or contributions