New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 26: The Hypothalamo-Pituitary System
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 26
|
 |
|
|
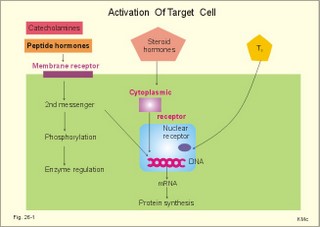
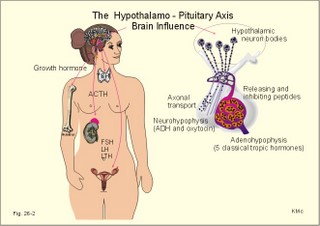
· To define autocrine, endocrine, neurocrine and paracrine transmission, endocrine feedback, endocytosis, exocytosis, hormones, hormone receptors, membrane receptors, neurohormones, neurotransmitters, phagocytosis, transcription, and tropic hormones. · To describe the structural relations between the brain, hypothalamus and hypophysis. To describe secretion mechanisms, second messengers, hormonal sensitivity, melanotropin secretion and function, and stimulation-secretion coupling. To describe the structure and function of hypothalamo-hypophyseal hormones, of tropic hormones from the adenohypophysis and of pro-opio-melano-corticotropin. To describe acromegaly, Cushings syndrome, gigantism, dwarf growth, panhypopituitarism, and hyperpituitarism. · To explain the secretion and function of growth hormone, somatomedins and somatostatin. To explain the secretion and function of gonadotropins, prolactin, relaxin, oxytocin, vasopressin. To explain hormonal function tests. · To use the above concepts in problem solving and case histories · The endocrine and nervous systems co-ordinate the functions of the other organ systems as regulators of the function of the whole body. · The endocrine system exerts its influence through blood-borne substances (hormones) produced in glands without secretory ducts (endocrine glands). · The endocrine glands comprise the hypothalamo-hypophyseal axis, which regulates the function of the thyroid, parathyroid, adrenal, and reproductive glands. Other important hormones are the growth factors, cytokines and gastrointestinal hormones. · Autocrine transmission refers to liberation and diffusion of signal molecules inside a cell to control functions in the cell of origin. · Calmodulin is a specific binding protein for Ca2+ inside cells. Different calcium-calmodulin complexes activate or inhibit the activities of calcium-dependent enzymes. · Calsequestrin is a specific binding protein for Ca2+ inside the sarcoplasmic reticulum of muscle cells. Calsequestrin is a buffer for cytosolic Ca2+-concentration. · Catecholamines are substances consisting of catechol (an aromatic structure with two hydroxyl groups) linked to an amine. The important catecholamines in humans are adrenaline, noradrenaline and dopamine. · Cushing’s disease is hypercorticism (increased glucocorticoid production) caused by a pituitary basophilic adenoma. · Cushing’s syndrome refers to the consequences of increased plasma glucocorticoid concentration from any source. · Cytokines are secreted polypeptides that affect the functions of other cells. · Domain is a segment of a protein molecule with a functional role independent of the rest. · Endocrine feedback is a system, whereby the first hormone, liberated to the blood stream, controls the secretion and liberation of the second. The second hormone acts by feedback and modulates the secretion of the first. · Endocrine transmission refers to transport of hormones along the blood stream to a distant target organ. · Endocytosis or pinocytosis refers to transport of molecules or material into the cell in vesicles of cell membrane. In some cases the coating is made by a surface protein called clathrin. Endocytosis requires metabolic energy (ATP). · Exocytosis is a process whereby the contents of intracellular vesicles (hormones, transmitters) are released to the external environment. · Feedback systems which are negative contain at least one step of inhibition. The total effect is to minimise any external change introduced to the system. Almost all hormone systems maintain homeostasis by negative feedback. · Feedback systems which are positive are systems, where an external change leads to increased secretion of hormone 1, which also leads to a secondary rise in hormone 2’s concentration. This is an auto-accelerating phenomenon and a rarity. · Hormones are messenger or signal molecules. Classical hormones are conveyed by the blood (endocrine substances) and their target cells are equipped with receptors that recognise each hormone. Hormone molecules form a large signal family together with neurotransmitters, and local diffusive (autocrine- paracrine) substances. · Hormone receptors are proteins, to which hormones bind, they are present in cell membranes, cytoplasm and nucleus, and serve two functions. Firstly, they are required for selectivity. Secondly, they are connected to an effector mechanism in the cell. In response to hormone binding, the receptor conformation is changed, and this activates a specific enzyme system that serves as an amplifier. · Membrane receptors are surface glycoproteins (just like immunoglobulins), that bind the water-soluble hormones (catecholamines and peptides). Some receptors have an amino acid sequence similar to a sequence within the hormone. · Neurocrine (neurosecretory) transmission refers to transport of a neurohormone first from the cell body of a neuron along its axon, and then with the blood to its target cells. · Neurotransmitters are signal molecules functioning in axonal transfer or between neurons. · Phagocytosis refers to transport into cells of bacteria and large foreign bodies. The cells a leucocytes and cells of the reticuloendothelial system that can destroy noxious substances. · Paracrine transmission is a release and diffusion of signal molecules with regulatory action on neighbour cells. · Radio-immuno-assays (RIA) refers to any method for detecting or quantitating antigens or antibodies utilising radiolabeled reactants. RIA utilises the competitive binding between a hormone and its induced antibody. RIA can be used to detect small quantities (high sensitivity), even in complex mixtures (high specificity). · Transcription (copying) is defined in Chapter 31 together with other genetic concepts. · Tropic hormones regulate the growth and hormone secretion from other cells. The five classical hormones from the adenohypophysis are tropic hormones. This paragraph deals with 1.Hormones in general, 2. Hormone receptors, 3. Monoamines, amino acids and peptides, 4. Radio-immuno-assays, 5. Hormone function tests, 6. Clinical application of hormones, 7. The hypothalamo-hypophyseal system, 8. The adenohypophysis, 9. The neurohypophysis. The scientists of the past used extirpation, substitution and transplantation to obtain the classical part of our present knowledge on endocrinology (ie, the discipline covering the internal secretion of signal molecules to the blood). Removal of the pancreas produced diabetes in animals. Removal of the pituitary gland, followed a few days later by removal of the pancreas, produced no symptoms of diabetes. This is because the adenohypophysis produces a hormone that is antagonistic to the effect of insulin in glucose metabolism. The hormone is human growth hormone. Houssay received a Nobel Prize for this work in 1947. Hormones are messenger or signal molecules. Classical endocrine hormones are secreted into the blood and transported to their distant target cells, which are equipped with receptors that recognise each hormone. The hormones co-ordinate the activities of different cells in order to maintain homeostasis and to secure growth and reproduction. Hormone molecules form a large signal family together with neurotransmitters, autocrine and paracrine acting substances. Paracrine and autocrine signal molecules are secreted and diffuse into the interstitial fluid surrounding the cells and their actions are restricted either to nearby cells (paracrine) or to the cell of origin (autocrine). Neurotransmitters (acetylcholine, adenosine, amines, amino acids, ATP, peptides) exert a type of paracrine action, since they are released in the synaptic region. All reactions in the cell linking stimulation and secretion together are termed stimulation-secretion couplings. Stimulation-secretion coupling involves depolarization of the cell membrane or opening of Ca2+ -channels, so that Ca2+ can diffuse into the cell and combine with its Ca2+-binding proteins. A rise in intracellular concentration [Ca2+] is necessary for exocytosis. Elimination of hormones takes place by metabolic processes such as the inactivation of peptide hormones by proteolytic enzymes, or the transformation of hormones in the liver. Hormones are also eliminated by excretion in the urine or bile. In the liver hormones are coupled to glucuronic acid or sulphate, but these hormones are in part reabsorbed in the entero-hepatic- circuit. Protein binding protects small hormone molecules (such as the thyroid hormone) from elimination. Protein binding also eases the transportation of the lipid-soluble steroids, and maintains equilibrium with a small free pool of hormone, so the concentration of free hormone is maintained. Hormones can be divided into three chemical categories: Peptides and proteins include neuropeptides, pituitary and gastrointestinal hormones. Steroids consist of adrenal and gonadal steroids and vitamin D, which is converted to a hormone. Steroids are lipid soluble (lipophilic). Monoamines (modified amino acids) comprise catecholamines, histamine, serotonin, and melatonin. Catecholamines (dopamine, noradrenaline and adrenaline) are derived from tyrosine - and serotonin/melatonin from tryptophan - by a series of enzymatic conversions. Monoamines and amino acid hormones are water soluble just as peptides. Thyroid hormones are iodinated derivatives of tyrosine, and thyroid hormones are lipophilic. The water-soluble hormones are packed in the Golgi complex in secretory granules that migrate to the cell surface. Exocytosis of the granule contents to the interstitial fluid (ISF) and diffusion through fenestrae to the capillary blood is a common method. The secretory cells are first stimulated by chemical or electrical signals. Synthesis of protein or peptide hormones takes place as outlined in Chapter 31. Transcription of the hormone gene results in a specific mRNA determining the synthesis of a single hormone. However, a single gene may dictate the synthesis of different peptides in different cells. As the signal protein is cut off, the prohormone is formed and transported to the Golgi apparatus and stored in granules. The hormone specific amino acid sequence is contained in the prohormone. An endocrine feedback system is a system whereby the first hormone controls the secretion and liberation of the second. The second hormone acts by feedback to modulate the secretion of the first. A negative feedback system contains at least one step of inhibition. The total effect is to minimise any external change introduced to the system. Almost all hormone systems maintain homeostasis by negative feedback. A positive feedback system exagerates any primary change initiated. - This is an auto-accelerating phenomenon and a rarity. The most important example in humans is the steep rise in blood [oestradiol] in the middle of the menstrual cycle. High [oestradiol], when maintained for longer than 35 hours, stimulates by positive feedback, the luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion from the adenohypophysis, which further stimulate oestradiol secretion etc. By contrast, moderate plasma [oestradiol] levels, which are present during the other parts of the cycle, provide negative instead of positive feedback. Long feedback systems act on the hypothalamo-pituitary system from remote target organs. Short feedback systems use a short distance feedback, such as the influence of the hypophysis back to the hypothalamus. Auto-feedback refers to the action of a liberated hormone that was secreted on the cell from where it came thereby modulating its own secretion. These are proteins, to which hormones bind. They are present in cell membranes, cytoplasm and nucleus, and serve two functions. Firstly, they are required for selectivity. Secondly, they are connected to an effector mechanism in the cell (Fig. 26-1). In response to hormone binding the receptor conformation is changed, and this activates a specific enzyme system that serves as an amplifier. In the cytosol, multiple second messengers have evolved to serve such purposes, whereas in the nucleus, the hormone-receptor complex binds to DNA and regulates gene expression (Fig. 26-1). The effector domain of the membrane receptor is directly coupled to the regulatory portion of the effector enzymes (such as adenylcyclase = adenylate cyclase). These effector enzymes control ion fluxes, membrane transport systems, the production of cyclic nucleotides, and the breakdown of phospholipids. Inactive kinases are activated by the use of ATP. This phosphorylation is critical for for transformation of information and for cell viability (synthesis, transport and metabolism of vital molecules). Many hormones initiate a series of reactions when bound to membrane receptors. One family of coupling molecules, called G-proteins, links some of the receptors to nearby effector molecules (see Chapter 1). Other receptors make use of another system. Fig. 26-1: Target cells activation by hormones acting at Membrane, Cytoplasmic, and Nuclear receptors. Steroids and thyroid hormones are lipophilic and therefore pass easily through the cell membrane by diffusion. Steroids bind to specific cytosol-receptor proteins that are then translocated into the cell nucleus where they reversibly bind to DNA (Fig. 26-1). Some unbound receptor proteins may even exist in the nucleus. The binding of the steroid-receptor complex to the specific gene modulates mRNA transcription. Tri-iodo-thyronine (T3) binds to nuclear receptor proteins, which then attaches to a thyroid response unit in the gene in a manner similar to that of steroid receptors. The result is increased mRNA formation (Fig. 26-1). Steroids and thyroid hormones frequently work in conjunction with each other (potentiate amplification of gene expression). Cell membrane and intracellular receptors can change their affinity and number. A specific ligand for a receptor is able to modulate the total number of this receptor. Increasing the concentration of the ligand (hormone, neurotransmitter, drug) often reduces the number of receptors (down-regulation), and other hormones recruit their own receptors at low concentrations (up-regulation). Maximal effects of hormones are generally observed at receptor occupancy of less than 50%. The myoepithelial cells (myometrium and breast) contain oxytocin receptors. Their number is up regulated by estrogens and down regulated by progesterone. The cardiac muscle contains nor-adrenergic receptors (b1). Both affinity and number of receptors is increased by thyroid hormone stimulation (T3/T4). Internalisation is the transport of hormone-receptor complex into the cell by an endocytotic vesicle. This is a means of terminating the action of the hormone. After destruction of the hormone by lysosomes, the receptor returns to the surface and is reused. 3. Monoamines, amino acids and peptides Such water-soluble hormones (first messengers) bind to hormone receptors on the lipid-rich plasma membrane. Peptide hormone and catecholamine receptors are membrane receptors with a binding domain located extracellularly and an effector domain intracellularly (Fig. 26-1). The second messengers involved are cyclic adenosine monophosphate (cAMP), cyclic guanosine monophosphate (cGMP), inositol trisphosphate (IP3), Ca2+, diacylglycerol (DAG) etc. The Ca2+-ion is an important second messenger. The Ca2+-influx to the cytosol is controlled by hormone receptor binding, neural stimuli or modified by other second messengers. Sutherland discovered cAMP and demonstrated its role as a second messenger in mediating body functions (Nobel Prize 1971). Increased activity of the sympathetic nervous system including release of adrenaline triggers fight-or-flight reactions. In the heart, adrenaline molecules diffuse to the myocardial cells, where they bind to membrane b-receptors. A stimulatory signal is hereby transmitted to an associated enzyme called adenylcyclase. This enzyme catalyses the conversion of ATP to cAMP. The importance of cAMP is that it activates protein kinase A, which, among many other functions, phosphorylates the Ca2+-channel protein. This activation is correlated with an increase in the magnitude of the Ca2+-influx, the force of contraction, and the heart rate. The parasympathetic system counteracts the sympathetic by slowing the heart rate and decreasing the force of contraction. Acetylcholine is bound to another set of specific membrane receptors located on the heart cell membrane. Acetylcholine reduces the Ca2+-influx that was increased by adrenaline. Most hormones have a blood concentration of approximately 10-10 mol per l. One molecule bound to a cell receptor releases 10 000 times more cAMP in the cell. Hence, cAMP works as an amplifier of the hormone signal. Phosphodiesterase (PDE) destroys cAMP. PDE enhances hydrolysis of cAMP to the inactive 5’- AMP by a highly exergonic process. Inhibitors of the PDE (theophylline and caffeine) act synergistically with hormones that use cAMP as a second messenger. cAMP stimulates catabolic processes such as lipolysis, glycogenolysis (glucagon), gluconeogenesis, and ketogenesis. The cAMP also stimulates amylase liberation in the saliva by the parotid gland, the HCl secretion by the parietal cells, the insulin release by the b-cells in pancreas, and the increased ion permeability of many cell membranes. When the glucose concentration increases in the arterial blood and close to the b-cells of the pancreatic islets of Langerhans, it triggers an increase in Ca2+ -influx to the cell. The initial surge in insulin secretion is caused by calmodulin-dependent protein kinases. The high cytosolic [Ca2+] activates the membrane phospholipase A2 and C. Phospholipase A2 releases arachidonic acid (AA) which stimulates insulin secretion. Phospholipase C catalyses the formation of IP3 and DAG. The IP3 releases more Ca2+ from the endoplasmic reticulum, and DAG activates protein kinase C. The decrease in insulin secretion after the initial surge and its subsequent increase can be explained by the action of protein kinase C. Initially, the active protein kinase C stimulates the Ca2+-pump in the plasma membrane, reduces cytosolic [Ca2+] and thus reduces the initial calmodulin-dependent insulin secretion. Later, protein kinase C stimulates the formation of cAMP and amplifies the induction of calmodulin-dependent protein kinase thereby causing a gradual increase in insulin secretion. Prolonged glucose stimulation probably leads to down-regulation of protein kinase C. An abnormally prolonged glucose stimulation may render b-cells glucose blind and thus spoil their function. Insulin secretion is not only stimulated by glucose, but also potentated by acetylcholine via phospholipase C and by glucagon via activation of adenylcyclase. b-Agonists stimulate b-receptors on the glucagon producing a-cells, whereas a-agonists inhibit insulin secretion via a2-receptors on the b-cells. Acetylcholine and glucagon react by activating protein kinase C and cAMP dependent protein kinase A, respectively. Both mechanisms potentate the Ca2+-triggered insulin secretion. Transcription in the cell nucleus produces a precursor messenger RNA molecule complementary to part of a DNA. The precursor is processed into messenger RNA and transported through the nuclear membrane into the cytoplasm. Messenger RNA carries the genetic information in triplet codons (Chapter 31). Messenger RNA binds to ribosomes and transfer RNA molecules synthesise peptides (ribosomal translation). Translation produces big precursor molecules (pre-pro-hormones). Precursors have a signal peptide that contains processing information to ensure that the protein enters the rough endoplasmic reticulum. Here enzymes split the precursor into a signal molecule and a prohormone. Finally, peptide hormones undergo post-translational processing (for eg, thyroid stimulating hormone, TSH, and gonadotropins are glycosylated; insulin forms a zinc-complex). The hormones reach the Golgi complex, where they are packed into secretory granules that migrate to the cell surface. Roger Guillemin synthesized brain peptides that regulate the pituitary secretion in vitro. He received the Nobel Prize in 1977. RIA refers to any method for detecting or quantitating antigens or antibodies utilising radiolabeled reactants. RIA is used to detect very small quantities of antigens or antibodies, even in complex mixtures. First a specific antibody is produced towards the antigen (eg, hormone). In one version of RIA for antigen detection, the antigen is radiolabeled and reacted with a limited amount of specific antibody. The complex containing bound antigen is then separated from free antigen. Unlabeled antigen in a test sample is used to compete with the binding of radiolabeled antigen. The test antigen is quantitated from the extent of inhibition obtained with standards containing defined amounts of the same antigen. Rosalyn S. Yalow and Saul Berson developed the RIA method. Rosalyn Yalow received the Nobel Prize in 1977. Recent variations of the RIA technique include immuno-radiometric, chemi-luminescent, and enzyme-linked radioimmuno-sorbent assays. - In the radioreceptor assay a hormone receptor is substituted for the antigen-antibody in RIA. The following tests are clinical tools in the diagnosis of hormone disorders: 5.1. The hormone concentration in the blood is commonly used. It can be measured by taking advantage of the new methods described above. 5.2. The secretion flux of T3 and T4 from the thyroid gland. 5.3. The metabolic rate or the absorption rate of 131I (radioactive iodine) in the thyroid gland. The physical half-life of 131I is 8 days or 192 hours. The elimination rate constant (k) of a substance is the amount eliminated per unit time divided by the total amount present in the distribution volume, assuming exponential elimination. The variable k is easy to calculate: T½ = ln 2/k = 0.693/k. The value of k for iodine is 0.693/192 or 0.0036 hours-1. 5.4. The elimination rate: Abnormal amounts of catecholamines or VMA (Vanillyl mandelic acid) in a 24-hour urine suggest the presence of a catecholamine-producing tumour (phaeochromocytoma). 5.5. Stimulation test: Stimulation with ACTH (Adrenocorticotropic hormone) without a substantial rise in plasma -[cortisol] suggests primary, adrenocortical atrophia. 5.6. Suppression test: Dexamethasone (a cortisol synergist) is administered to a Cushing suspect patient in the evening. The next morning a measurement of plasma [cortisol] shows suppression in normal persons and in patients with a primary, adrenocortical hyperfunction. The cortisol synergist reduces ACTH secretion and thus cortisol production by negative feedback. Hypothalamic/pituitary Cushing is never suppressed by cortisol. 5.7. The glucose tolerance test: A load of glucose normally triggers an increased rate of insulin production. 6. Clinical applications of hormones Distribution of oestrogens and progesterone in contraceptives (P pills) is world-wide. Oestrogens are widely used to relieve postmenopausal discomfort. Now some females with osteoporosis are treated experimentally with calcitonin, because calcitonin inhibits osteoclastic bone resorption. Insulin is a lifesaver for diabetics, and it is produced and distributed as pure human insulin. In the affluent areas of the world many women deliver their babies following an oxytocin infusion. Oestrogens and gonadotropins are used in treatment of sterility and menstrual disturbances. Huggins received the Nobel Prize in 1966 for the introduction of a new form of cancer therapy in which sex hormones are used to retard their growth. He used androgens for breast cancer and oestrogens for prostate cancer. 7. The hypothalamo-hypophyseal system. The human pituitary gland consists essentially of two parts both controlled by the hypothalamus. The glandular part is the adenohypophysis or anterior lobe, and the neural part is the neurohypophysis or posterior lobe. The adenohypophysis develops ectodermally from the primitive mouth cavity (Rathkes pouch). Blood-borne signal molecules from the hypothalamus regulate the cells of the adenohypophysis. The neurohypophysis develops from the neuro-ectoderm in the floor of the third ventricle. The two parts combine to form one body called the adeno-neuro-hypophysis that weighs about 0.5 g. The infundibular process of the neurohypophysis receives blood from the inferior hypophyseal artery, whose capillary plexus drains into the adenohypophysis (Fig. 26-2). The upper stalk and the median eminence is supplied with blood by the carotid artery to the superior hypophyseal artery, whose primary capillary plexus ends in long portal veins carrying blood to the highly permeable secondary capillary plexus of the adenohypophysis. From this plexus, blood drains into the dural sinus. The adenohypophysis lies outside the blood-brain barrier, and does not receive arterial blood directly. A third capillary plexus, between the neurohypophysis and the median eminence of the hypothalamus, allows short loop feedback from the hypophysis to the hypothalamus. The hypothalamus and the hypophysis connects in the following ways (Fig. 26-2): 1. The neurosecretory axons pass from the cell bodies in the supraoptic and paraventricular nuclei of the hypothalamus to the neurohypophysis. The neurosecretory granules are here stored in the terminals of these axons. The granules are released by exocytosis upon stimulation. The peptides from the granules then enter the capillary plexus of the inferior hypophyseal artery 2. The hypophysiotropic zone in the median eminence of the hypothalamus is connected to the adenohypophysis. Both releasing and inhibiting peptides are synthesized in hypothalamic neuron bodies and transported to the median eminence in granules via axonal transport. At the median eminence the inhibitory and releasing signal molecules are discharged to the capillary plexus of the superior hypophyseal artery. From here they follow the blood through the long portal veins to reach the specific cells in the adenohypophysis. Here, the releasing and inhibiting hormones modulate the output of tropic hormones. Fig. 26-2: The hypothalamo-pituitary axis. The 5 classical tropic hormones are corticotropin, gonadotropins (FSH, LH), somatotropin, thyrotropin and mammotropin. Neurosecretory neurons (which have nuclei in the hypothalamus and axons that lead to the median eminence and to the posterior lobe of the hypophysis) and peptidergic neurons (spread in the nervous system and gut) produce and liberate peptides in much the same way. The secretory granules travel through the axons of the neurosecretory neurons that form the supraoptico-hypophyseal tract, with high velocity (more than 100 mm each hour). This tract runs through the pituitary stalk and end in the neurohypophysis. The transfer is known as axoplasmic transport. The neuro-hypophyseal terminals are located close to the capillary blood. During transport the pro-hormone splits into its subunits. Oxytocin and vasopression are then released. The nerve endings of this tract in the neurohypophysis are the storage area for these two neurosecretory hormones; secretion to the blood takes place through fenestrated capillaries. The secretion granules release their content by regulated exocytosis. Exocytosis is triggered when the neurosecretory neuron is depolarised and an action potential is transferred to the terminals. Even a small rise in the osmolarity of plasma stimulates osmoreceptors, located close to the neurosecretory cells in the hypothalamus. The osmoreceptors stimulate both production and secretion of vasopressin (ADH) in the neurosecretory cells. The plasma [ADH] will then rise from the basal level that is 2 pmol per l. The normal secretion flux is 10-13mol ADH per kg body weight per min, and the biological half-life in human plasma is 18 min. Some females increase their plasma [ADH] in the pre-menstrual phase. The neuroregulatory peptides are endogenous opiates (endorphins and enkephalins), b-lipotropin, neurotensin, substance P, VIP or vasoactive intestinal peptide etc. Many of these peptides are cut off from a big mother molecule: pro-opio-melanocortin (POMC). These peptides may exhibit a permissive effect on other hormones (ACTH, growth hormone) related to behaviour and autonomic responses. During exercise (a Cooper test which lasts 12 min) the plasma [b-endorphin] and [ACTH] increases by 200-300% from the normal resting averages of 1.7 and 2.2 pmol per l, respectively. There is an increase in plasma [ACTH] and its accompanying neuroregulatory peptides during prolonged stress like exhaustive exercise and chronic disease. The endogenous opiates affect stress-adapting behaviour, such as the euphoria observed in the chronically ill. The pituitary gland normally has a mass of approximately 500 mg, but it increases during pregnancy and decreases with aging. The five hormones from the adenohypophysis are tropic hormones - they regulate the growth and hormone secretion of target cells (Fig. 26-2). They include: 1. Thyrotropin or thyroid-stimulating hormone (TSH), which is produced in thyrotropic cells, 2. Gonadotropins (FSH and LH) from gonadotropic cells, 3. Corticotropin (ACTH) from corticotropic cells, 4. Somatotropin (human growth hormone, HGH or GH) from somatotropic cells and 5. Prolactin or mammotropin produced in mammotropic cells. The five tropic peptide hormones have the following molecular characteristics: 1. One group contains glycoproteins with two peptide chains. There is a special, biologically active b-chains for each of the three hormones TSH, FSH and LH, although the inactive a-chain is the same for all of them. 2. Somato-mammotropins are single-chain peptides containing 200 amino acids of almost the same sequence. HGH and prolactin (PRL) are probably simple gene duplicates from the same prohormone molecule. 3. POMC peptides are neuroregulatory hormones: ACTH, endogenous opiates, b-endorphin, b-lipoprotein, a-MSH and b-MSH (MSH abbreviates melanocytic stimulating hormone). Histamine plays an important role in pituitary hormone secretion. Histamine stimulates the secretion of ACTH, b-endorphin, a-MSH, and PRL. Histamine participates in the release of these hormones during prolonged stress and possibly in the suckling- and oestrogen-induced PRL-release. The release of growth hormone (GH) and TSH are predominantly inhibited by histamine. GH is the main stimulator of body growth in humans (Chapter 30). Histamine increases the secretion of LH in females - mediated by GnRH (gonadotropin releasing hormone). Histamine probably affects the cell bodies in the supraoptic and paraventricular nuclei, stimulating the formation of arginine vasopressin and oxytocin. The neurohypophysis secretes two hormones: vasopressin and oxytocin. Vasopressin or antidiuretic hormone (ADH) is a vasopressor with a strong antidiuretic effect as the names imply. Vasopressin is normally synthesized as a big pre-prohormone in the ribosomes of neurons in the supraoptic and paraventricular nuclei of the hypothalamus. The pre-prohormone consists of a signal peptide, ADH, neurophysin and a glycopeptide. First, the signal peptide is cut off, and then the precursors are packed in secretion granules in the Golgi complex. The secretion granules travel by axoplasmic transport through the axon of the neurosecretory neurons that form the supraoptic-hypophyseal tract, and then are stored in its terminals in the neurohypophysis. These terminals are located close to the fenestrated capillaries. The smallest rise in the osmolarity of plasma stimulates osmoreceptors located close to the neurosecretory cells of the hypothalamus. The osmoreceptors stimulate both production and release of ADH. Vasopressin is a nonapeptide with a molecular weight of 1084 Da. ADH has the following effects: 1. ADH eases the renal reabsorption of water in the cortical collecting ducts (and not in the outer medulla but in the inner medulla) - leading to antidiuresis. 2. ADH probably stimulates the active solute reabsorption (NaCl) in the thick ascending limb of the renal Henle loop. Thus, ADH helps maintain the concentration gradient in the kidney. 3. Vasopressin is a universal vasoconstrictor. Vasopressin reduces the small, medullary bloodflow through vasa recta along the Henle loop. ADH acts on the basolateral membrane of the cells, and the result is a rise of [cAMP] in the cytosol. The cAMP diffuses to the luminal side, where it causes vesicular structures to develop and fuse with the luminal membrane. Hereby, the membrane receives a large number of water channels, so the membrane becomes highly water permeable. Water diffuses through the cell to the basolateral membrane and into the interstitial fluid. Stimulation of tactile receptors in the mammary nipple causes the neurosecretory neurons to release oxytocin through a neuroendocrine reflex. The latency between the stimulus and milk ejection is due mainly to the transport of oxytocin in the blood from the neurohypophysis to the milk ducts (20-30 s). Oxytocin stimulates the myoepithelial cells in the milk ducts of the lactating breast so that milk is ejected to the baby. Oxytocin also stimulates the myoepithelial (myometrial) cells of the uterus satisfying the woman sexually during breast-feeding. Oxytocin can perhaps start labour. · This paragraph deals with the five tropical hormones from the adenohypophysis and vasopressin secreted from the neurohypophysis: 1.Pituitary TSH-secreting tumours, 2. Polycystic ovarian syndrome, 3. Basophilic pituitary adenoma, 5. Prolactinomas, 6. Diabetes insipidus, 7. Syndrome of inappropriate ADH secretion, 8. Panhypopituitarism. 1. Pituitary TSH-secreting tumours A pituitary TSH-secreting tumour is an extremely rare cause of thyrotoxicosis. Thyrotoxicosis is dealt with in Chapter 28. 2. Polycystic ovarian syndrome Chaotic LHRH secretion from the hypothalamus to highly sensitive gonadotropic cells in the pituitary increases the LH-level in the blood plasma. Actually, LHRH induces its own receptors. The gonadotropin level is so high in the follicular phase that androgens in excess are produced from the theca cells. These females produce immature or atretic follicles occurring as multiple cysts in enlarged ovaries. A maintained LH-level can be produced by other causes. The excessive gonadotropin and androgen secretion causes irregular bleedings, subfertility, acne and hirsutism. 3. Basophilic pituitary adenoma Basophilic pituitary adenoma is the cause of classical Cushing’s disease. The excessive ACTH secretion induces adrenocortical hypersecretion of cortisol. The hyper-cortisolaemia causes the many symptoms and signs found in Cushing’s syndrome (Chapter 30). 4. Somatotropic pituitary adenoma Somatotropic pituitary adenomas produce large amounts of growth hormone leading to gigantismus in childhood and to acromegaly in adults. These cases of hyperpituitarism are dealt with in Chapter 30. In rare cases the cause is excessive GHRH secretion from the hypothalamus. Some acromegalics also produce excess prolactin in hypertrophic mammotrophs. Pituitary adenoma cells with TRH receptors also secrete excess GH. TRH is used as a diagnostic test. Other pituitary adenoma cells have somatostatin receptors. Somatostatin and somatostatin agonists inhibit GH secretion, and make the adenomas shrink Prolactinomas are microlactinomas (less than 1 cm), which cause anovulatory, irregular bleedings, abnormal milk production (galactorrhoea), and subfertility. The constantly elevated plasma prolactin inhibits the LH-secretion necessary for ovulation. – Dopamine, dopamine agonists and somatostatin analogues inhibit prolactin secretion and can make the prolactinomas shrink. The true form of diabetes insipidus is caused by deficiency of vasopressin (ADH deficiency). There are two types of diabetes insipidus. The primary or idiopathic type, which is due to a genetic defect that blocks the hormone production, and the secondary type, where the hypothalamo-hypophysary system is damaged by disease or surgery. The renal tubule cells are rarely insensitive to ADH, and this infrequent condition is called renal diabetes insipidus or nephrogenic diabetes insipidus. This is a sex-linked recessive disorder or it is acquired from renal disorders or hypercalcaemia. The symptoms and signs are mainly due to the large diuresis (polyuria), nocturia, and a tremendous thirst (polydipsia). A total lack of ADH can result in a diuresis of 25 l daily. ADH eases the renal reabsorption of water in the cortical collecting ducts and in the inner medulla via a cAMP mechanism which increases the number of water channels in the luminal membrane. ADH stimulates NaCl reabsorption in the thick ascending limb of Henle, and vasopressin is a universal vasoconstrictor. Synthetic vasopressin is given intra-nasally as a spray up to 3 times daily. 7. Syndrome of inappropriate ADH secretion ADH producing tumours in the hypophysis or in the lungs causes the syndrome of inappropriate ADH secretion. Water retention, concentrated urine, hyposmolar plasma, and muscle cramps characterise this syndrome. is typically due to total destruction (lesions or tumour invasion) of all hormones in the hypothalamo-hypophysary system. Lack of GH and somatomedins result in a dwarf without normal sex development (lack of LH and FSH). This dwarf has also hypothyroidism (lack of TSH), and a Cushing-like syndrome (hypercorticism without excess ACTH secretion). The differentiation of somatotrophs, mammotrophs and thyrotrophs is dependent upon a protein transcription factor (Pit-1). Mutation of the Pit-1 gene leads to hypoplasia of the adenohypophysis and to insufficient production og GH, prolactin and TSH with hypopituitarism. The following five statements have True/False options: A. High-pressure liquid chromatography is a sensitive analysis used for many hormones and biochemical key molecules. B. he affinity and number of specific membrane receptors on a given cell is constant. C. Monoamines and amino acid hormones are water-soluble just as peptides. D. cAMP inhibits catabolic processes such as lipolysis, glycogenolysis, gluconeogenesis, and ketogenesis. E. Hyperpituitarism is often caused by microadenomata, which typically cause dwarf growth. The adenohypophysis of a 23 year old woman contains approximately 300 mg of TSH with a molecular weight of 31 000. TSH has a half-life (T½) in plasma of 55 min and a concentration of 100 pmol per l of plasma. The haematocrit of the patient is 0.5. The woman secretes TSH to her total blood volume (TBV), which is 4 l. 1. Develop an equation for the calculation of her TSH secretion (J mol/hour). The rate constant k can be used. 2. Calculate the secretion of TSH from her adenohypophysis 3. What fraction of her total TSH store is secreted per 24 hours? A woman (24 years of age; height: 1.70 m; weight: 60 kg) is in hospital due to a tremendous thirst, and she drinks large amounts of water. Since she is producing 10 or more litres of urine each day, the doctors suspect the diagnosis to be diabetes insipidus. The vasopressin concentration in plasma (measured by a RIA method) is 10 fmol per l. Her secretion of vasopressin is only 5% of the normal flux of 10-13 mol per min per kg body weight. The normal plasma [vasopressin] is 2 pmol per l as a mean. The extracellular volume (ECV) is 20% of her body weight. Vasopressin is injected intravenously at several occasions. A dose of 3 mg vasopressin is the minimum necessary to normalise her diuresis for 4 hours. Before the injection her diuresis is 6 ml of urine per min, but within 25 min her urination is constantly around 0.5 ml/min. 1. Calculate the secretion of vasopressin (in mg/hour) from the neurohypophysis of a normal 60-kg person and of this patient. 2. Calculate the distribution volume for vasopressin, which is 20% higher than ECV. 3. Assume the 3 mg vasopressin injected to be distributed evenly immediately after the intravenous injection. Calculate the rise in vasopressin concentration in the distribution volume. 4. Estimate the relation between this concentration and that of a healthy individual. 5. Does this ratio have implications for the interpretation of her special type of diabetes insipidus? 6. Is it dangerous to lose 10 litres of urine per day? Try to solve these problems before looking up the answers . · The hypothalamo-pituitary system controls the function of the adrenal, thyroid, and reproductive glands, as well as regulating growth, lactation, milk secretion and water excretion. · Protein and peptide hormone synthesis starts with processing of a primary gene transcript (code) called a prohormone. The processing includes proteolysis, glycosylation and phosphorylation. · Catecholamines, peptide and protein hormones are stored in secretory granules and discharged by exocytosis. · Catecholamines, peptide and protein hormones are water-soluble and cannot pass the cell membrane. They act on the surface of target cells via membrane receptors. The hormone-receptor-complex activates second messengers in the cell (cAMP, Ca2+, DAG, IP3) via stimulatory or inhibitory G-proteins. · Thyroid and steroid hormones are lipid-soluble and act through specific nuclear receptors. The hormone-receptor-complex modulates elements in DNA molecules in order to change the expression of target genes. · Peptide hormones produced in the cell bodies of hypothalamic neurons pass down their axons inside secretory granules to be stored in the terminals of the neurohypophysis. · Releasing and inhibiting peptides from the hypothalamus are released in pulses in the adenohypophysis and act via second messengers. They modulate transcription, translation and secretion of tropic hormones. · Vasopressin or antidiuretic hormone is a vasopressor with a strong antidiuretic effect as the names imply. Vasopressin (ADH) is normally synthesized as a big pre-prohormone in the ribosomes of neurons in the supraoptic and paraventricular nuclei of the hypothalamus. The pre-prohormone consists of a signal peptide, ADH, neurophysin and a glycopeptide. · POMC peptides are neuroregulatory hormones: ACTH, endogenous opiates, b-endorphin, b-lipoprotein, a-MSH and b-MSH. · Stimulation of tactile receptors in the mammary nipple causes the neurosecretory neurons to release oxytocin through a neuroendocrine reflex. Oxytocin stimulates the myoepithelial cells in the milk ducts of the lactating breast. Oxytocin also stimulates the myoepithelial (myometrial) cells of the uterus. Oxytocin can perhaps start labour. · The true form of diabetes insipidus is caused by deficiency of vasopressin (ADH deficiency). There are two types of diabetes insipidus. The primary or idiopathic type, which is due to a genetic defect that blocks the hormone production, and the secondary type, where the hypothalamo-hypophysary system is damaged by disease or surgery. · ADH producing tumours in the hypophysis or in the lungs causes the Syndrome of inappropriate ADH secretion. Water retention, concentrated urine, hyposmolar plasma, and muscle cramps characterise this syndrome. · Panhypopituitarism is due to total destruction (lesions or tumour invasion) of all hormones in the hypothalamo-hypophysary system. Lack of GH and somatomedins result in a dwarf without normal sex development (lack of LH and FSH). This dwarf has also hypothyroidism (lack of TSH), and a Cushing-like hypercorticism (wothout ACTH secretion). · Hyperpituitarism is often caused by prolactin producing microadenomata, which cause abnormal milk production. This leads to disturbance of the menstrual cycle and infertility. Other pituitary adenomas produce large amounts of GH leading to gigantismus in childhood and to acromegaly in adults. Nature. Weekly journal published by Macmillan Magazines Ltd, Porters South, 4 Crinan Street, London N1 9XW, UK. Cell. Bi-weekly journal published by Cell Press, 1050 Massachusetts Av., Cambridge Massachusetts 02138, USA. Yalow, R. S. “Radioimmunoassay: Historical aspects and general considerations”, in Handbook of Experiment. Pharmacol., 1987.
|
||
Click here to introduce your comments or contributions