New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 32: Immunology and Immune System Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 32
|
 |
|
|
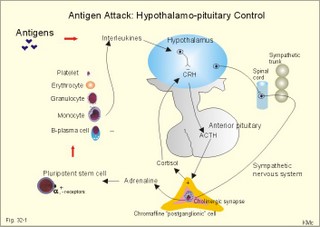
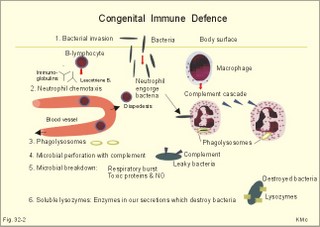
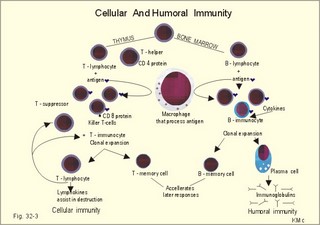
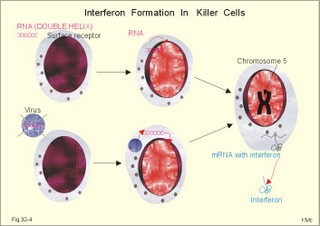
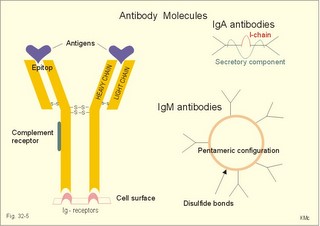
· To define the following concepts and disorders: AIDS, allergy, autoimmune reactions, HIV, interleukins, neutropenia, the reticulo-endothelial system (RES), serum disease, and vaccination. · To describe anaphylactic reactions, the role of natural killer cells, of soluble lysozymes, active and passive immunisation. · To explain the mechanisms involved in allergy, acquired and congenital immunity, haemopoiesis, phagocytotic killing, myasthenia gravis, ulcerative colitis and Crohns disease, rheumatoid arthritis, insulin-dependent diabetes mellitus, pernicious anaemia, and Graves hyperthyroidism. · To use the above concepts in problem solving and case histories. · The immune system defends the body against invading agents, participates in autoimmune and hypersensitivity disorders, and determines transplant tissue reactions. · The ability to recognise foreign antigens allows destruction and removal of invading organisms by various effector mechanisms. · Inappropriate immune reactions against self-antigens or host cells result in autoimmune disorders. · Overt responses to an antigen result in hypersensitivity disorders. · Acquired Immuno-Deficiency Syndrome (AIDS) results from infection with Human Immuno-Deficiency Virus (HIV). This is a substantial T-lymphocyte defect. The HIV is bound to receptors on CD4+ T-lymphocytes, but also to monocytes and macrophages. · Autoimmune disorders occur when the immune system kills the body’s own cells or self-antigens, because the system fails to recognise them. Autoantibodies are produced and immune complexes are deposited in the tissues. Here, complement and neutrophils are activated and accumulated. The neutrophils release proteolytic enzymes and toxic cytokines. · Delayed allergic reactions occur when highly resistant allergens (mycobacterium such as TB, fungi, contact allergens) get into contact with T-lymphocytes, and do not involve production of antibodies. Repeated contact with antigen activates helper T- and killer T-cells. · Interleukins are toxic lymphokins and monokines from lymphocytes and monocytes. · Natural killer cells destroy tumour cells and virus-infected cells. They are unspecific, non-phagocytotic lymphocytes that are activated by interferon produced by the affected cell. Interferon induces a high degree of resistance in the affected cell. · Neutropenia are too few neutrophils in the peripheral blood. · The reticuloendothelial system (RES) is also called the mononuclear phagocytotic system (MPS). Lymphoid organs belonging to the RES are the bone marrow, the liver, the spleen, the lymph nodes, the microglia of the brain, the thymus, tonsils, as well as MALT, BALT, GALT, and SALT (see below). · Serum disease results from passive immunisation with too many antibodies. Antigen-antibody complexes accumulate in the blood. They precipitate in the slow bloodstream of the capillaries. · Soluble lysozymes are enzymes in plasma, lymph, extracellular fluid, saliva, gastric fluid and other secretions, which can destroy the bacterial wall. · Vaccination is iatrogenous immunity. At the first vaccination with a certain antigen, some plasma cells transform to memory B-cells that remain in the RES. At the second vaccination, the memory B-cells evoke an exaggerated antibody production that rapidly deactivates the antigens. This paragraph deals with 1. The immune system, 2. Congenital immunity, 3. Acquired immunity, and 4.Vaccination. Burnet received the Nobel Prize in 1960 for his clonal selection theory. Pluripotent stem cells differentiate into millions of different B-lymphocytes, T-lymphocytes, erythrocytes, polymorphonuclear leucocytes, monocytes, macrophages and mast cells. Lymphocytes are the most important cells involved in immune responses. Without exposure to all the antigens each of the B-lymphocytes have inherited the ability to divide into a clone of plasma cells. The first contact with the specific antigen starts the clone production. The clone of plasma cells produces the specific immunoglobulins. This understanding of the immuno-reaction made transplantation possible. Thomas made the first transplantation of kidneys in 1956, and received the Nobel Prize in 1990 for his contribution to science and therapy. The second successful transplantation was the transplantation of bone marrow to treat leukaemia (ie, uncontrolled proliferation of impotent leucocytes). Overactivity in the immune system causes allergic and autoimmune disorders, whereas underactivity results in immunodeficiency. The immune system is a complex of cells and humoral factors controlled by the hypothalamo-hypophysary axis in concert with the adrenal and probably other endocrine glands (Fig. 32-1). The major organs of the reticuloendothelial system, RES (bone marrow, lymph nodes, spleen, and thymus) receive sympathetic efferents - in particular the T-lymphocyte regions (see below). Hereby, the CNS (via the hypothalamus) modulates the intensity of immunoreactions. Endotoxins from the normal bacterial flora of the intestine constantly enter the blood. Ordinarily they are inactivated by phagocytic activity of the RES mainly in the liver. Macrophages not only inactivate endotoxins; they also release hydroxylase, proteases, certain coagulation factors and arachidonic acid derivatives (ie, prostaglandins, thromboxanes, leucotrienes and monokines). Monokines are control proteins that modulate metabolism (Chapter 20), temperature control (Chapter 21), hormone secretion (Chapter 26), and the immune defence systems. The important role of RES in haemorrhagic and endotoxic shock is described in Chapter 12. Fig. 32-1: Control of the immune system by the hypothalamo-pituitary axis during an antigen attack. The immune system protects us against disease. The system confers congenital (inborn) and acquired immunity. Both subsystems activate soluble humoral factors as well as fixed and mobile cells. Congenital immunity involves T-lymphocytes, which are derived from the thymus, whereas acquired immunity involves B-lymphocytes and the production of antibodies. The bone marrow is the site of haemopoiesis, since all blood cells are derived from the pluripotent stem cell or haemocytoblast (Fig. 32-1). This is a primitive cell type, which can divide rapidly and differentiate into committed stem cells. The committed stem cells are colony- forming in that they are committed to produce large quantities of erythrocytes, granulocytes (neutrophils, eosinophils and basophils), monocytes-macrophages, megacaryocytes-blood platelets, and B- & T-lymphocytes (Fig. 32-1) depending upon various growth inducers or cytokines. Interleukines are toxic lymphokines and monokines from lymphocytes and monocytes. They inhibit the hypothalamic production and release of corticotropin-releasing- hormone (CRH) just as cortisol - thus reducing the immuno-response. Cortisol also inhibits lymphocyte and monocyte production from stem cells (Fig. 32-1). Normally, CRH stimulates the synthesis and release of adrenocorticotropic hormone (ACTH). Stimulation of the sympathetic nervous system by immunological stress releases adrenaline from the adrenal medulla. Adrenaline probably stimulates most of the blood cell formation from stem cells (erythro- granulo- lympho- monocyto- thrombo- poiesis) via a2-adrenergic receptors. The inborn immune defence system is unspecific and responsible for immediate responses to infection (bacteria, fungi, parasites, and viruses) and other pathogens (from tumours or other sources). The inborn system is immediately activated with all the elements of congenital immunity: Phagocytes (neutrophils and macrophages), cytotoxic eosinophils, histamine-containing basophils and mast cells, and essential proteins (complement, acute phase proteins, heat shock proteins). Phagocytes comprise a large number of neutrophils, which are released from the bone marrow during acute infection. Neutrophilic granulocytes have an extremely short life cycle, namely 24 hours. They are leucocytes formed in the bone marrow. The production of neutrophils is increased by the action of granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage-colony stimulating factor (GM-CSF). During severe long-lasting infections the bone marrow is exhausted and too few neutrophils are released to the blood (ie, neutropenia). Neutrophils are important in the defence against microorganisms. Fig. 32-2: Congenital immune defence against bacteria. Granulocytes can leave the blood by moving between endothelial cells to reach the interstitial space of different tissues. 2.1. Steps of microbial destruction Bacterial invasion. When bacteria invade the body, macrophages release the complement cascade, and B-lymphocytes release immunoglobulins (Fig. 32-2). Neutrophilic chemotaxis. Complement cascade products and leucotriene B4 (see later) are released from cells in the infection area. These molecules attract neutrophils from the blood into the infected tissue by so-called chemotaxis (ie, attraction of cells by foreign chemical substances). The neutrophils pass the endothelial wall by diapedesis (ie, they squeeze through the capillary wall - see Fig. 32-2). Neutrophils surround the microbe with their pseudopodia and engorge them. Neutrophils are large enough to phagocytize bacteria and fungi, but they cannot phagocytize larger organisms such as parasites. Phagolysosomes. The pseudopodia form a membrane bound vesicle around the microbe, and the vesicle is then released as a free-floating phagosome. Inside the neutrophil, the phagosome fuses with neutrophil granules to form phagolysosomes, where the killing occurs. Phagocytes get hungry from opsonization of the pathogen surface with complement or with specific immunoglobulins such as IgM and IgG. Microbial perforation. The complement released from many macrophages also fights its own battle. Besides being bound to immunoglobulins, complement is also bound to the surface of bacteria, whereby they get leaky. Microbial breakdown. Phagocytotic killing occurs in the phagolysosomes. The method of execution is by a respiratory burst or by gas. Oxygen is reduced to reactive oxygen metabolites by an NADPH oxidase. These reactive metabolites are hydrogen peroxide and oxygen radicals. Many toxic proteins or enzymes (lipases, proteases) take part in the destruction. Immuno-stimulated macrophages produce nitrite and nitrate, and their killer activity is related to the unstable gas, nitric oxide (NO). NO is produced in large quantities by the macrophages, kills microbes and cancerous cells. NO is synthesized from one of the guadino nitrogens of L-arginine by the enzyme nitric oxide synthase. Several synthases have been purified and cloned. The enzymes represent a new family that contains a haeme moiety. Soluble lysozymes are enzymes in plasma, lymph, extracellular fluid, saliva, gastric fluid and other secretions, which can destroy the bacterial wall (Fig. 32-2). Specific antibodies and complement cascade substances ease the execution of microbes. Neutrophils carry receptors for immunoglobulins and complement on their surfaces, which increase the binding force between the cell and the microbe, and simultaneously transduct signal molecules to increase the enzymatic killing activity. This is a typical co-operation between congenital and acquired immunity. The capillaries in the area dilate and get leak for proteins. This is why the site of invasion gets hot, red, swells, and pains (Latin: calor, rubor, tumour, and dolor -the classical signs of inflammation). 2.2. Cytotoxic eosinophilia Eosinophils contain granules with substances, which become cytotoxic, when they are released on the surface of parasites. Thus, eosinophils are mainly involved in reactions against parasitic infections. Eosinophils are not phagocytic, but they intoxicate nematodes and other parasites and bacteria. The cytotoxic substances are major basic protein, which kill helminths, eosinophil cationic protein (an extremely efficient killer of parasites and potent neurotoxins) and eosinophil peroxidase (kills bacteria, helminths and tumour cells). Eosinophils are involved in hypersensitivity reactions. 2.3. Histamine containing cells Circulating basophils and mast cells residing in the tissues are morphologically similar with granules that contain histamine and other vasoactive amines. These histamine containing cells are involved in hypersensitivity reactions (see IgE-mediated allergy). The binding of IgE to the cells stimulate the release of histamine, but also of prostaglandins, leucotrienes and cytokines. These substances cause immediate (Type I) hypersensitivity. The T- mast cell contains trypsin and cytoplasmic IgE, and the TC- mast cell contains both trypsin and chymotrypsin. 2.4. Natural killer cells Such cells destroy tumour cells and virus-infected cells. They are unspecific, non-phagocytotic lymphocytes that are activated by interferon produced by the affected cell. Interferon induces a high degree of resistance in the affected cell. 2.5. Essential proteins Complement extirpates microbes and immune complexes. The complement system includes several serum glycoproteins that are activated in a cascade similar to the coagulation cascade (Chapter 8). Complement activation destruct and removes microbes, immune complexes and tumour cells, recruits cells and proteins to infection sites by chemotaxis, and modulates the B-cell immune response. Acute phase proteins (C-reactive protein, complement complex, fibrinogen, haptoglobulin, caeruloplasmin, a1-antitrypsin) are produced in response to infection and inflammation (ie trauma, necrosis, tumours etc). The disease activity is measured in blood serum as C-reactive protein. Heat shock proteins preserve the protein structure of cells during infections. They resemble antigens and are involved in immunity and autoimmunity. Antigen stimulation of inactivated lymphocytes results in development of humoral- or cell-mediated immune responses. Humoral responses involve antibodies from B-lymphocytes activated to large antigen-producing plasma cells. Also macrophages and T-helper cells are required. Cell-mediated responses require cells that produce antigens and cytokines to T-helper cells. Some pathogens can prevent phagocytosis or suppress the formation of lysosomes or kill the neutrophils. When attacked by such pathogens we must rely on acquired or specific immunity. This is produced by rearrangements of germ-line DNA in B- and T-lymphocytes. Hereby, specific antibodies and specific antigen-binding T-cell receptors are created. Tonegawa has shown how a rearrangement of DNA in only a few genes can produce millions of different antibodies in an individual. This is enough to cover all antigens encountered. In foetal life, cells from the bone marrow pass through the gastrointestinal lymph nodes. Here the inactive cells become immunologically active B-lymphocytes. The cells re-enter the blood and migrate to the foetal spleen, liver and other lymph nodes. When an antigen binds to receptors on these cells, the lymphocytes divide, and from now on the whole clone of plasma cells can produce the specific antibody. 3.1. The reticuloendothelial system (RES) This system is also called the mononuclear phagocytotic system (MPS). Lymphoid organs belonging to the RES are the bone marrow, the liver, the spleen, the lymph nodes, the microglia of the brain, the thymus, tonsils, as well as MALT, BALT, GALT, and SALT (see below). These organs contain macrophages originating from monocytes. Inactivated and circulating macrophages are called monocytes, but when they nitigate into extravascular tissues they are known as macrophages. Macrophages contain lysosomes filled with various catabolic enzymes. The macrophage membrane contains receptors for binding complement components and immunoglobulins. Macrophages destroy other phagocytized organisms or molecules by production of free radicals and digestive enzymes. Tumour necrosis factor (TNF) is produced by macrophages stimulated by bacterial cell wall components. TNF turns a tumour into haemorrhagic necrosis. Recombinant TNF is available in the form of TNF-a and TNF-b (lymphotoxin). The cell content of the RES organs covers fixed and locally wandering macrophages as well as B-lymphocytes, which produce the antibodies after antigen exposure, and are now called plasma cells. B-lymphocytes comprise 25% of all lymphocytes. The remaining lymphocytes (75%) are T-lymphocytes, which are undergoing a maturation process in the thymus. T-lymphocytes possess distinct cell surface antigens. RES receive sympathetic efferents. Hereby, the hypothalamus can modulate the intensity of immunoreactions. This is what is termed the psycho-immune coupling process (Fig. 32-1). The spleen is the largest lymphoid organ in the body containing both B- and T-lymphocytes. The lymph nodes are distributed all over the body. The thymus contains cells that originate from the bone marrow. The lymphocytes derived from the thymus are called T-lymphocytes. The immature T-lymphocytes are matured to CD4+ and CD8+ by thymic hormones. The thymus also deletes cells that are reactive to the body's own tissues (clonal deletion). MALT, BALT, GALT, and SALT are lymphoid tissues found in the intestinal mucosa (mucosa-associated lymphoid tissue = MALT), in the wall of the main bronchi (bronchus-associated lymphoid tissue = BALT), in the gut (gut-associated lymphoid tissue = GALT), and in the skin (skin-associated lymphoid tissue = SALT). Tonsils combat airborne antigens by the help of antigen producing B-lymphocytes. Fig. 32-3: Formation of sensitised lymphocytes, lymphokines and antibodies. B-lymphocytes are involved in acquired, humoral immunity, and T-lymphocytes in congenital, cellular immunity. 3.2 T-lymphocytes acquire their immune competence in the thymus (Fig. 32-3). They are divided into helper T-cells and killer T-cells. Helper T-cells carry CD4 protein on their surface and produce lymfokines (interferon and interleukin-2 and -4). Helper T-cells help the killer T-cells to proliferate, to destruct antigen and to reinforce antibody production. Some external antigen molecules are processed in macrophages before they bind to the lymphocytes (Fig. 32-3). Helper T-cells activate resting B-lymphocytes, so they differentiate to plasma cells or to active B-lymphocytes. Some new cells develop to plasma cells and remain in the lymph nodes. Interleukin-2 is a peptide of 133 amino acid moieties. This substance stimulates the production of lymphokine-activated killer cells that destroy tumour cells without affecting normal cells. Interleukin-3 stimulates the primitive stem cell. Interferon is called so, because they interfere with viral RNA and protein synthesis; interferon probably induces enzymes that destroy viral RNA and other proteins. Human can produce at least 3 types of interferon (a, b, g); they are glycoproteins with a molecular weight of 20-25 kD. With viral or RNA stimulation, a-interferon is synthesized in macrophages (Fig. 32-4) and b-interferon in fibroblasts and macrophages. The g-interferon (no sequence homology to the two other forms) is produced in antigen-stimulated T-lymphocytes. The g-interferon stimulates the antigen production in macrophages and B-lymphocytes. Recombinant interferon is commercially available. Interferon is used in the treatment of severe attacks of condylomata acuminata, chronic hepatitis B or C, and certain types of sarcomata. Fig. 32-4: Production of interferon in macrophages following stimulation with RNA or virus. T-lymphocytes constitute the majority of blood lymphocytes. The lymphocytes proliferate at first contact between antigen and T-lymphocytes. Some new cells bind the antigen in an antigen-antibody reaction and destroy the antigen. Killer T-cells is the proper name for these cells, but the destruction of antigen requires the co-operation of helper T-cells. Helper T-cells stimulate the proliferation and differentiation of killer T-cells to increase their number. A subgroup of effector T-cells can suppress antibody formation by B-lymphocytes and inhibit other effector T-cells. the so-called suppressor T-cells. Congenital immunity is a delayed form of immunity. The response reaches a peak after 2 days. Delayed immunity reaction encompasses the rejection of transplants, contact allergies and defence reactions against certain viruses and fungi. The T-cell number is deficient in AIDS victims (Acquired Immune Deficiency Syndrome). The T-lymphocytes recognise self-antigens, known by the body's own cells and non-self antigens from cancer cells, foreign cells (transplantates) and foreign molecules (external antigens). This recognition ability is acquired early in life, when lymphoid stem cells migrate into the thymus, where a few are modified into memory T-cells and released to the blood. At the first contact between antigen and T-lymphocyte the cell proliferates. Some of the new lymphocytes are killer T-cells. They bind the antigen in an antigen-antibody complex and destroy the antigen. Killer T-cells carry CD8 protein on their surface and kill other cells suffering from cancer or virus infection. Some of the killer T- cells are actually suppressor T-cells, because they can suppress antibody formation by the B-lymphocytes and inhibit other effector T-cells. Hereby they can down-regulate the immune response when necessary to prevent autoimmune responses. 3.3. B-lymphocytes produce specific antibodies or immunoglobulins to antigens (Fig. 32-3). The immunoglobulins form part of the gamma-globulin fraction in plasma. The B-lymphocytes multiply by clonal expansion. A specific antigen is bound and recognized to the B cell through special surface immunoglobulins. B cells also contain CD19 and CD20 proteins. Cytokines activate the B-lymphocyte, so it divides and the resulting cells differentiate to enormous plasma cells with an overwhelming surplus of protein-producing endoplasmic reticulum (ER). This is why plasma cells produce large amounts of antibodies and release them into the blood as Y-shaped molecules (Fig. 32-3). The plasma cells have a short life cycle, and die when they have fulfilled their defence mission. Hereby, the B-lymphocyte population is reduced to its normal size apart from a few cells remaining as memory cells. The antibodies are also called immunoglobulins (Ig). They are specific serum glycoproteins. Each antibody is Y-shaped and consists of heavy and light polypeptide chains. The heavy chains with complement receptors provide the constant domain of the Ig molecule, which is the same in all antibodies (Fig. 32-5). The light chain region constitutes the variable domain, which is functionally important. Antibodies deactivate antigens by forming a complex, which causes agglutination and precipitation, by masking the active sites of the antigens, or by activating the complement cascade. A single Ig with its antigen activates a complement cascade with mobilisation of up to 109 new complement molecules carrying lots of enzymes that rapidly lyse the antigen-carrying microbe. The most abundant is IgG, which has a high antigen affinity and is the antibody of the secondary response to protein antigens (viruses and tetanus toxin). IgG can cross the placental barrier and protect the newborn for a couple of months. IgM is confined to the blood, because it is a pentameric molecule (5 IgM molecules joined together). IgM cannot cross the placental barrier, and is responsible for the primary immune response. IgA1 predominates in serum, whereas IgA1 and IgA2 are present in equal amounts in secretions such as saliva, gastric juice, pancreatic and intestinal juice. IgA protects mucosal surfaces in the gut, respiratory and urinary tracts, by preventing the attachment of poliovirus, enterovirus, bacteria, and enterotoxin. The concentration of IgD in serum is high in disorders with B-lymphocyte activation such as AIDS and Hodgkin's disease. IgE is mainly bound to basophils and mast cells, and involved in the pathogenesis of allergic and nematode diseases. Fig. 32-5: An immunoglobulin (Ig) or antibody molecule with two antigen molecules attached (left). The immunoglobulins IgA and IgM are build up of two or more immunoglobulins moieties connected with disulphide bonds (right). This is iatrogenous immunity. At the first vaccination with a certain antigen, some plasma cells transform to memory B-cells that remain in the RES. At the second vaccination, the memory B-cells evoke an exaggerated antibody production that rapidly deactivates the antigens. Vaccine from death microbes is used for bacterial diseases such as diphtheria and typhoid fever. Other vaccines are derived from toxins that are deactivated without losing their antigen specificity (tetanus, botulism). Vaccines against viral disease have passed through a series of other organisms, until a mutant originates without pathogenic activity but with intact antigen specificity (measles, polio, smallpox, and yellow fever). Tumour-antigen vaccines are under development in order to stimulate an immune reaction against tumour cells. This paragraph deals with 1.Congenital and acquired immuno-deficiencies due to underactivity of the immune system, and 2. Allergic and autoimmune diseases caused by overactivity of the immune system. 1. Congenital and acquired immuno-deficiencies The 5 major types of congenital immuno-deficiency are:
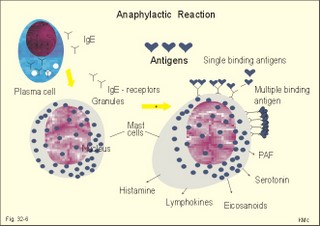
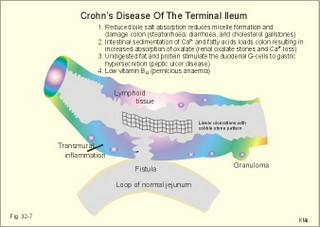
Acquired immunodeficiency (AID) Iatrogenic AID is caused by splenectomy, chemotherapy or iatrogenically induced malnutrition. Glucocorticoids suppress immune responses and the movement of lymphocytes from the blood to the tissues. Autoimmune suppression by infection is Acquired Immuno-Deficiency Syndrome. AIDS results from infection with Human Immuno-deficiency Virus (HIV). This is an overwhelming T-lymphocyte defect. The HIV is bound to receptors on CD4+ T-lymphocytes, but also to monocytes and macrophages. HIV and AIDS are described in Chapter 33. Allergic and autoimmune disorders are typically hypersensitivity reactions. Allergic reactions are either immediate anaphylactic (type I) or delayed (type II). Autoimmune conditions are also called type III hypersensitivity reactions. 2.1.Immediate anaphylactic reactions (type 1) occur immediately when the allergen sensitises B-lymphocytes with allergen specific IgE antibodies (ie, within minutes). At the next exposure to the same allergen, plasma cells recognise the allergen and release large amounts of IgE (Fig. 32-6). The allergen-IgE complex is bound to IgE-receptors on the surface of the mast cells and basophils. Hereby, histamine, serotonin (a vasoconstrictor), lymphokines, platelet activating factor (PAF) and toxic eichosanoids (prostaglandins and leucotrienes) are released (Fig. 32-6). PAF activates both thrombocytes and phagocytes. Histamine is a powerful vasodilatator and bronchoconstrictor. Leucotrienes were previously called slow reacting substances for anaphylaxis (SRS-A). Leucotrienes are strong bronchoconstrictors causing bronchial asthma attacks. Cells participating in inflammatory and immune responses are suppressed by prostaglandin E2 (PGE2). When PGE2) stimulates adenylcyclase in activated neutrophils, they release less leucotriene (LTB4) than else; the same mechanism is probably functioning in other cells. Atopic allergy is genetic and characterized by large amounts of IgE in the blood. An antigen molecule with multiple binding sites can bind to many IgE molecules on the mast cell and release large amounts of anaphylactic substances (Fig. 32-6). Three typical disorders are the following: a. The so-called anaphylactic shock is often fatal shortly after the histamine release. Adrenaline injected intravenously or intracardially may save the victim. b. Urticaria and hay fever are anaphylactic reactions in the skin and the nasal mucosa, respectively. The histamine released causes vasodilatation, with increased capillary pressure and ultrafiltration causing oedema and red colour. c. Bronchial asthma in an atopic person is a bronchiolar anaphylactic response. Leucotrienes from the bronchiolar mast cells cause bronchoconstriction, mucosal infiltration with inflammatory cells, mucosal oedema and hypersecretion. Leucotrienes are blocked by a variety of bronchodilatators. Fig. 32-6: Anaphylactic reaction as it occurs in mast cells and basophils. 2.2. Type II: Delayed allergic reactions occur when highly resistant allergens (mycobacterium such as TB, fungi, contact allergens) get into contact with T-lymphocytes, and do not involve production of antibodies. Repeated contact with antigen activates helper T- and killer T-cells. These cells diffuse into the skin, lungs or other organs and there release a cellular immune response. 2.3 Type III: Autoimmune disorders occur when the immune system kills the body’s own cells or self-antigens, because the system fails to recognise them. Autoantibodies are produced and immune complexes are deposited in the tissues. Here complement and neutrophils are activated and accumulated. The neutrophils release proteolytic enzymes and toxic cytokines. They may kill cells in A) a single organ system or B) affect multiple systems. 2.3.A) Single organ disorders: Insulin-dependent diabetes mellitus (IDDM). Newly presenting patients possess islet-cell antibodies that have destroyed all the insulin producing b-cells of the pancreatic islets (Chapter 27). There is an inherited increased susceptibility to IDDM. Pernicious anaemia. Typically parietal cell antibodies are found in the blood. They may kill the entire parietal cell population leading to atrophy of the mucosa. The atrophic gastric mucosa fails to produce hydrochloric acid and intrinsic factor for vitamin B12 (Chapter 8). Also intrinsic factor antibodies have been found, either able to block the complex binding between intrinsic factor and vitamin B12, or capable of blocking the binding of the intrinsic factor and vitamin B12 -complex to its ileal receptors. Graves's or Basedow's disease is hyperthyroidism combined with eye signs (exophtalmus). Normally, the thyroid stimulating hormone (TSH) increases the thyroid hormone production after its binding to the thyroid TSH receptors. Bacterial infection in a genetically susceptible person may be the cause of autoimmune production of the TSH-receptor antibodies. These IgG -antibodies behave exactly like TSH itself, and thus stimulate the thyroid hormone production (Chapter 28). Retroorbital swelling and damage of the extraocular muscles cause the thyroid eye disease from specific antibodies. Atrophic hypothyroidism is caused by microsomal autoantibodies in the thyroid. The hypersensitivity reactions result in thyroid atrophy and hypothyroidism (Chapter 28). Hashimoto's thyroiditis is caused by other microsomal autoantibodies. The inflammatory reaction produces goitre (struma) with or without hypothyroidism. Primary hypo-adrenalism (Addison's disease). The entire adrenal cortex is destroyed by organ-specific autoantibodies, and its steroid hormone production (sex hormones, glucocorticoids, and mineralocorticoids) is lost (Chapter 30). Autoimmune disease is the cause of destruction in 4 of 5 patients. Other causes are TB, cancer, infections, and AIDS. Myasthenia gravis. This is a rare disease caused by autoantibodies against the acetylcholine receptors of the neuromuscular endplate (Chapter 2). Complement complexes and IgG molecules are deposited at the endplates. Many patients have thymic hyperplasia. Crohns disease and ulcerative colitis These two disorders may be different manifestations of a single disease, non-specific inflammatory bowel disease. Ulcerative colitis is a disorder located to the colon only, whereas Crohns disease can involve any part of the gastrointestinal tract. Fig. 32-7: Inflammatory bowel disease (Crohns disease). Transmissible agents are suspected but not found. Both antigen specific, cellular and autoimmune responses have been postulated. The immune-hypersensitivity is evident from activation of T-lymphocytes, neutrophils, eosinophils, basophils and mast cells. A variety of cytokines, Eicosanoids, oxygen radicals and NO are produced. Dysfunction of the terminal ileum has serious consequences, because bile salts are not reabsorbed and vitamin B12 is not absorbed. The cause of inflammatory bowel disease is unknown, but some features are common to the two conditions: 1. Autoimmune disease in the gut lumen and wall. 2. Cytotoxic T lymphocytes sensitised by antigens destroy the mucosa or the whole gut wall. 3. Genetic predisposition (concordance in monozygotic twins, familial occurrence), 4. Infective agents are suspected but not identified. 5. Uveitis, episcleritis, arthritis, anchylosing spondylitis, erythema nodosum occur in both types of bowel disease. 6. The clinical picture includes abdominal pain and diarrhoea often with blood and mucus. Steatorrhoea typically points to ileal involvement, whereas frequent bloody diarrhoeas indicate colic involvement as in ulcerative colitis. There is an increased risk of colorectal carcinoma in ulcerative colitis. Crohns disease is a chronic infection or inflammation of the gut with a particular prevalence for the terminal ileum, but it can be located all the way along the tract. The patients with terminal ileitis suffer from malabsorption of bile salts and vitamin B12. Ulcerative colitis is always confined to the colon and it is a mucosal inflammation with haemorrhage and rectal bleeding. Special hospital units accomplish different diagnosis and management. Gluten-sensitive enteropathy or coeliac disease (sprue) describes a condition where the duodenal and jejunal mucosa is more or less destroyed by hypersensitivity towards gluten. Gluten is found in barley, rye, wheat, and oats. Gluten consists of 4 gliadin peptides out of which a-gliadin destroys the jejunal mucosa. There is a sequence homology between a-gliadin and adenovirus 12. Nontropical sprue. In allergic persons gluten causes an immunological reaction with desquamation of the luminal part of the intestinal mucosa - in particular most of the microvilli. The marked fall in area available for absorption causes malabsorption. In severe cases the malabsorption involves fats causing steatorrhoe, Ca2+ causing osteomalacia, vitamin K causing bleeding disturbances, vitamin B12 causing pernicious anaemia, and folic acid causing folic acid deficiency. The inheritance is unknown, but this is a disease occurring in atopic families, and an immunogenetic mechanism is likely - in particular after infection with adenovirus 12. Symptoms and signs include stomatitis with ulcers, diarrhoea, steatorrhoea, abdominal pain, osteomalacia and malnutrition with oedema. The desquamation of the mucosa sometimes descends along the villous region in the small intestine and thus includes the terminal ileum. A gluten-free diet is necessary for successful treatment. Tropical sprue is found in tropical areas, and probably caused by gastrointestinal infection, although the bacterial diagnosis is seldomly confirmed. Tropical sprue is often curable with antibacterial agents. 2.3.B) Multiple system disorders: Rheumatoid arthritis (symmetrical inflammatory polyarthritis with progressive joint damage and lung, cardiac, renal and many other organ manifestations) is an autoimmune disease. The synovial fluid contains IgG, lymphokines and immune complexes. The cause is probably a persistent external antigen, which is not removed. Connective tissue diseases are Systemic Lupus Erythematosus (SLE = Arthralgia, rash, cerebral and renal dysfunction), systemic sclerosis (sclerosis of the skin and oesophagus, organ fibrosis, Raynaud's phenomenon), polymyositis (necrosis of muscle fibres with proximal weakness), dermatomyositis (polymyositis with rash) and other rare disorders. The aetiology is unknown, but many of these conditions have circulating autoantibodies and immune complex deposition. Several autoimmune disorders show a strong association. Each of the following five statements have True/False options: A. The pluripotent stem cell is not identical with the haemocytoblast. B. Interferon induces a high degree of resistance in the affected cell. C. The hypothalamus can modulate the intensity of immunoreactions through sympathetic efferents to the RES. D. Hyperthyroidism is always combined with exophtalmus. E. The terminal ileum does not have essential functions. A female, 39 years of age, works as a secretary. For 6 months she has been working with a new copy machine. Over the last three months she has developed a blistering rash on both hands and the rash is now turned into deep scaling of the skin. There is a mild scaling at the site of both her earrings. The patient has used a bland cream without effect. The dermatologist examines her with patch tests, where solutions of common allergens, chemicals, metals including nickel are placed on her back and occluded with dressings. The next day (24 hours later) the dressings are removed and the exposed areas examined. The examination is repeated 48 hours after the beginning of the exposure. There is a severe rash at the site of the colour powder used in the copy machine, and a mild rash at the nickel spot. 1. What is the diagnosis? 2. Which therapeutic strategy is recommendable? Try to solve the problems before looking up the answers. · The immune system is a complex of cells and humoral factors controlled by the hypothalamo-hypophysary axis in concert with the adrenal and probably other endocrine glands. · The inborn immune defence system is unspecific and responsible for immediate responses to infection (bacteria, fungi, parasites, and viruses) and other pathogens (from tumours or other sources). · Congenital immunity involves T-lymphocytes, which are derived from the thymus, whereas acquired immunity involves B-lymphocytes and the production of antibodies. · The bone marrow is the site of haemopoiesis, since all blood cells are derived from the pluripotent stem cell or haemocytoblast, which can divide rapidly and differentiate into committed stem cells. · The committed stem cells are colony- forming in that they are committed to produce large quantities of erythrocytes, granulocytes (neutrophils, eosinophils and basophils), monocytes-macrophages, megacaryocytes-blood platelets, and B- & T-lymphocytes depending upon various growth factors or cytokines. · Anaphylactic shock is often fatal shortly after the histamine release. Adrenaline injected intravenously or intracardially may save the victim. · Urticaria and hay fever are anaphylactic reactions in the skin and the nasal mucosa, respectively. The histamine released causes vasodilatation, with increased capillary pressure and ultrafiltration causing oedema and red colour. · Bronchial asthma in an atopic person is a bronchiolar anaphylactic response. Leucotrienes from the bronchiolar mast cells cause bronchoconstriction, mucosal infiltration with inflammatory cells, mucosal oedema and hypersecretion. Leucotrienes are blocked by a variety of bronchodilatators. · Iatrogenic AID is caused by splenectomy, chemotherapy or iatrogenically induced malnutrition. · Autoimmune suppression by infection is the Acquired Immuno-Deficiency Syndrome (AIDS) resulting from infection with Human Immuno-Deficiency Virus (HIV). This is a substantial T-lymphocyte defect. · Immuno-stimulated macrophages produce nitrite and nitrate, and their killer activity is related to the unstable gas, nitric oxide (NO). NO, produced in large quantities by the macrophages, kills microbes and cancerous cells. · Phagocytotic killing occurs in the phagolysosomes. The method of execution is by a respiratory burst or by gas. · Myasthenia gravis. This is a rare disease caused by autoantibodies against the acetylcholine receptors of the neuromuscular endplate. Complement complexes and IgG molecules are deposited at the endplates. Many patients have thymic hyperplasia. · lcerative colitis and Crohns disease (transmural enteric ulceration with a particular affinity for the terminal ileal cells) are of unknown aetiology, but they may represent two aspects of the same disease. Both antigen specific, cellular and autoimmune responses have been postulated. · Rheumatoid arthritis (symmetrical inflammatory polyarthritis with progressive joint damage and lung, cardiac, renal and many other organ manifestations) is an autoimmune disease. The synovial fluid contains IgG, lymphokines and immune complexes. The cause is probably a persistent external antigen, which is not removed. · Insulin-dependent diabetes mellitus (IDDM). Newly presenting patients possess islet-cell antibodies that have destroyed all the insulin producing b-cells of the pancreatic islets. There is an inherited increased susceptibility to IDDM. · Pernicious anaemia. Typically parietal cell antibodies are found in the blood. They may kill the entire parietal cell population leading to atrophy of the mucosa. The atrophic gastric mucosa fails to produce hydrochloric acid and intrinsic factor for vitamin B12. · Graves's or Basedow's disease is hyperthyroidism combined with eye signs (Exophtalmus). Normally, the thyroid stimulating hormone (TSH) increases the thyroid hormone production after its binding to the thyroid TSH receptors. Bacterial infection in a genetically susceptible person may be the cause of autoimmune production of the TSH-receptor antibodies. These IgG -antibodies behave exactly like TSH itself, and thus stimulate the thyroid hormone production. Scandinavian Journal of Immunology. Monthly journal published by Blackwell Science Ltd., Osney Mead Oxford OX2 OEL, UK. Scientific American. Monthly journal published by Scientific American Inc., 415 Madison Avenue, N.Y., USA. Mims C, Playfair J, Wakelin D, and R Williams. Medical Microbiology. 4th Ed. Mosby, London, 2007.
|
||
Click here to introduce your comments or contributions