New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 10: Cardiac Performance And Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 10
|
 |
|
|
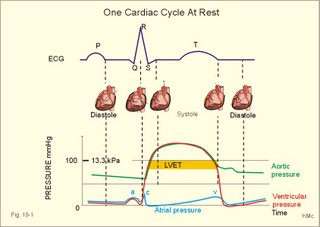
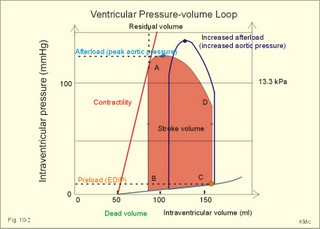
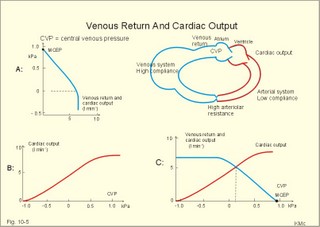
· To define concepts such as cardiac insufficiency, central venous pressure, compliance, contractility, venous pump, venous return and ventricular stroke work. · To describe four capacities of the cardiac pump called bathmotropic, chronotropic, inotropic, and dromotropic states. To describe the pressures of the left and the right half of the heart. To describe atherosclerosis, arteriosclerosis and risk factors involved. · To draw pressure-volume curves and contractility lines for the heart at different conditions, and pressure variations in the left side of the heart and the aorta. · To calculate the external work on the blood by the right and left ventricle, and the kinetic energy. · To explain the autonomic innervation of the heart, venous return, cardiac contraction, Starling´s law of the heart, cardiac performance and oxygen demand, and cardiac filling pressure. To explain ischaemic heart disease, rheumatic heart disease, Buerger´s disease, and Raynaud´s disease. · To use the above variables and concepts in problems and case histories. · Starling´s law of the heart: With an increased ventricular filling during diastole (venous return), the ventricular fibre length increases, so the ventricular contraction and stroke volume increases. - This is an intrinsic adaptation of the pumping capacity to the venous return. - Starling’s law of the heart is also called the Frank-Starling relationship, because it was described independently by Otto Frank and Starling. · The Fick principle (cardiac output) states that the volume of oxygen taken up by the blood in the lungs, divided by the arteriovenous oxygen content difference, is equal to the cardiac output. This example utilises the law of conservation of matter. · The law of Laplace - Eq. 8-6 · Poiseuille´s law - Eq. 8-3. · Angina pectoris (chest pains) is pain felt beneath the sternum or in the precordial area. The hypoxic pains are typically provoked by exercise or cold and submitted by subendocardially situated nerve fibres. The pains are relieved rapidly by nitro-glycerine and rest. · Arteriosclerosis refers to atherosclerosis (and further changes) of the peripheral arteries. · Atherosclerosis is a process of progressive lipid accumulation (atheromatosis) and calcification of the inner arterial walls in the abdominal aorta, lower extremities and the arteries of the heart, brain and kidneys. · Bathmotropic state refers to the irritability of the myocardium. · Cardiac insufficiency is a disorder, where the heart cannot pump enough blood to satisfy the nutritive needs of the body. · Central venous pressure (CVP) is the pressure measured in the caval veins at the level of the heart or in the right atrium. · Chronotropic state refers to the cardiac frequency. · Compliance of the resting cardiac chambers refers to dV/dP (chamber compliance) - the reverse of the elastance (dP/dV) of relaxed tissue. · Cardiac contractility is the dP/dV of the contracting ventricle. The contractility is depicted on the pressure-volume loop of the cardiac ventricle. Contractility refers to the change in slope of the pressure and volume increase from isovolumetric rest to contraction. Contractility is a function of contraction by crossbridge cycling. · Ejection fraction refers to the stroke volume of blood as a fraction of the end-diastolic ventricular volume. This is a useful index of contractility. · Inotropic state is another term for the myocardial contractility. · Intermittent claudication refers to chronic ischaemia of the legs with hypoxic pains while walking. · Dromotropic state refers to the conduction velocity of the myocardial conduction system. · Maximum oxygen uptake is the oxygen uptake during maximum exercise. This is a measure of endurance capacity, and when expressed per kg of body weight it is also called the fitness number (ml min-1 kg-1). · Mean circulatory equilibration pressure (MCEP) is the filling pressure everywhere in the circulatory system following cardiac arrest. · Venous pump refers to all local external forces acting on valvular veins and facilitating venous return. · Venous return is the bloodflow reaching the right atrium (in steady state a similar bloodflow reaches the left atrium). · Ventricular stroke work is the work applied to the blood at each ejection from the ventricle. This paragraph deals with 1. Cardiac electro-mechanics, 2. Pressure-volume work, 3. Venous return, 4. The venous pump, 5. The lipoprotein metabolism, and 6. Risk factors. The heart is a four-chambered double pump. Every 24 hours the heart is ejecting more than 104 l of blood, and contracting more than 105 times. The total amount of work performed over the lifetime of a person is enormous. The order of size is calculated at the end of this chapter. The cardiac cycle describes volume, pressure, and electric phenomena in the left ventricle as a function of time, and one heartbeat is shown below (Fig. 10-1). The red clock-shaped curve is the intraventricular pressure. The left ventricle is closed to the aorta in diastole and blood flows from the atrium to the left ventricle (Fig. 10-1). The ECG is explained in Chapter 11. Contraction or ventricular systole results in closure of the mitral valve (Fig. 10-1). Fig. 10-1: Electro-mechanical events in the cardiac cycle. The aortic pressure is shown with a green curve. The blue atrial pressure curve has a, c, and v waves. For the ECG see Chapter 11. The systole is isovolumetric (ie, the volume of blood in the ventricle is unchanged) until the intraventricular pressure exceeds the aortic pressure. Then the aortic valve opens and ventricular ejection occurs (see thick upward arrow in Fig. 10-1). Bulging of the cuspidal mitral valve into the left atrium during isovolumetric contraction causes a rise in left atrial pressure (see the c-wave in the thin a-c-v-curve of Fig. 10-1). The intraventricular pressure reaches a plateau around 15-16 kPa and then begins to decrease. The aortic valve closes when the intraventricular pressure falls below aortic pressure (see small arrow indicating retrograde flow in aorta in Fig. 10-1). This is the end of the ejection phase or the left ventricular ejection time (LVET). The electrocardiogram (ECG) does not reflect the mechanical performance of the heart, but closure of the aortic valve corresponds in time with the end of the T-wave in ECG (Chapter 11). The a-wave occurs during the contraction of the right atrium by which it squeezes out extra blood just before ventricular systole (early atrial contraction). As the atrium relaxes, the CVP is reduced (blue atrial curve in Fig. 10-1). The next atrial wave is the c-wave, and this wave is produced by closure of the cuspidal valves and by the right ventricular contraction, because the increased ventricular pressure is transmitted backwards to the right atrium and large veins. Filling of the atrial chambers with blood is aided by the large atrial compliance. This is why the atrial pressure only rises modestly. The third atrial wave is the v-wave for venous return. Throughout ventricular systole and isovolumetric relaxation, venous blood returns to the heart, but the tricuspid valve is closed, so that the central veins and right atrium are distended. The coinciding pressure build-up is relieved, when the tricuspid valve opens at the start of diastole, and the pressure is reduced (Fig. 10-1). The increase in right atrial pressure is transmitted backwards to the large veins near the heart. Prominent waves can often be seen in the neck veins when supine. The periods just described for the left heart can be shown to be the same in the right heart, except for the fact that the systolic pressures are considerably lower in the right ventricle and pulmonary artery. The stroke volumes of the two ventricles are the same. Contraction of the right ventricle begins just after that of the left side and lasts for a shorter time (the peak pressures obtained are much less). At the end of the ventricular diastole the left atrial pressure increases, because the atrial systole begins just before the ventricular systole. The intraventricular pressure-volume loop is a time-independent representation of the cardiac cycle, where the instantaneous intraventricular pressure and volume is plotted (Fig. 10-2). In diastole from B to C, the ventricle receives blood from the left atrium. The small increase in ventricular pressure reflects passive expansion and elastance of the myocardial wall. Pressure and volume increase with a slope that is related to contractility. The green line represents minimal contractility. The line starts from the ventricular dead volume, that is a virtual minimal volume of blood, which can never be ejected (50 ml in Fig. 10-2). A steep rise in pressure occurs from C to D, with no change in ventricular volume (the isovolumetric contraction). At D the aortic orifice opens, because the end-diastolic pressure in the aorta is passed. During the rapid ejection phase, the fall in ventricular blood volume, is accompanied by a continuous increase in pressure. During ejection the volume falls by a size equal to stroke volume, pressure rises and falls until the residual ventricular volume is attained (about 80 ml in Fig. 10-2). The last ejection phase is slow, because the pressure decreases towards A, where the aortic orifice closes. The last event from A to B is the isovolumetric relaxation with a sharp drop in pressure at constant volume (Fig. 10-2). The steep slope of the curve refers to optimal contractility at a given condition (Fig. 10-2). Actually, the precise contractility concept is the change in slope from isovolumetric rest to the highest slope of the curve. Fig. 10-2: The left ventricular pressure-volume loop from a healthy person at rest. – The pressure-volume loop is a widely applicable pathophysiological tool. The area of the loop represents the pressure-volume work on the stroke blood volume performed by the ventricular contractile elements during ejection. The pressure in C is the end-diastolic intraventricular pressure or the so-called preload. The force against which the ventricle contract is termed afterload. A good index of the afterload is the peak aortic (or intraventricular) pressure during systole, equal to the highest pressure shown in Fig. 10-2. The afterload is almost equal to the peak systolic pressure in the arterial tree. When the afterload is increased at constant end-diastolic pressure and volume, a greater ventricular pressure develops in order to expel blood (the dashed curve from D in Fig. 10-2). The result is a smaller stroke volume (and hence a greater residual ventricular volume), because of the high aortic and intraventricular pressure (Fig. 10-2). The Frank-Starling relationship states that increasing left ventricular end-diastolic volume increases the stroke volume of the next heart beat. During isovolumetric contraction, the end-diastolic intraventricular pressure (EDIP) or preload increases. Increasing end-diastolic volume increases the ventricular filling pressure and thus stroke volume and stroke work. An increase in afterload occurs when the aortic pressure increases. Such an increase causes a decrease in stroke volume. Hereby, the EDIP and the end-diastolic volume increase, so the cardiac stroke work increases concomitantly (Fig. 10-2). The ventricular stroke work is the sum of the pressure-volume work and the kinetic work and calculated according to Eq. 10-1. Preload is the end-diastolic filling pressure of the ventricle just before contraction (C and E in Fig. 10-3).
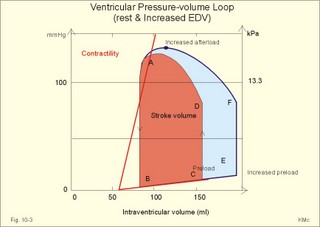
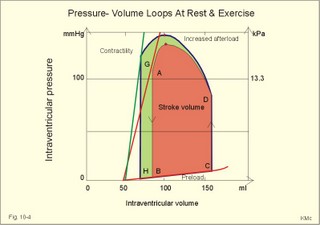
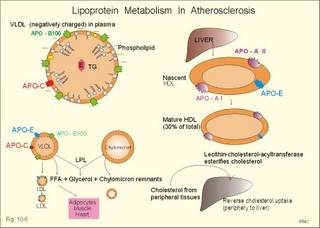
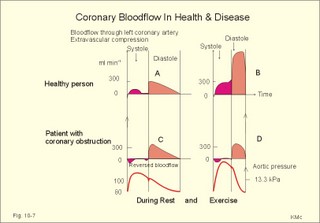
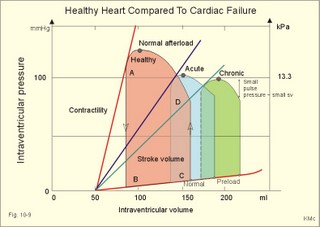
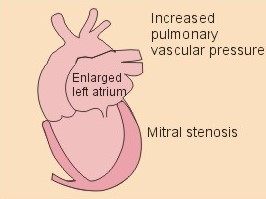
Fig. 10-3: Left ventricular pressure-volume loops at rest (red area) and following an increase in end-diastolic volume by increased diastolic filling (blue loop with E-F). When the left ventricle expands from C to E, by receiving more blood than before, the end-diastolic volume is increased. The greater diastolic filling results in a larger stroke volume according to Starling’s law of the heart. The Frank-Starling relationship can be formulated as follows: Any increase of preload (ventricular filling) invokes a progressive increase in the blood volume ejected by the ventricles beat-by-beat until the cardiac output equals the input.The ventricular pressure increases with the rise in systolic aortic pressure (increased afterload in Fig. 10-3). The stroke work of the heart on the blood is the area A, B, E, F, A. Accordingly, the stroke work is increased. A sympathetically mediated increase in contractility without a change in end-diastolic pressure (preload) results in an increased intraventricular pressure (Fig. 10-4). The slope of the line through G illustrates the increased contractility. The larger stroke volume, smaller residual volume, and larger stroke work on the blood (area C, D, G, H) is also shown in Fig. 10-4. Fig. 10-4: Left ventricular pressure-volume loops from a healthy person at rest and following an increase in contractility during exercise (G-H). The transmural pressure rises during ventricular contraction even at constant fibre tension. This is because the short ventricular radius is reduced to the same extent (see the law of Laplace, Eq. 8-5). Echocardiography shows that the short ventricular radius is reduced by 5-6 mm during systole in healthy people at rest. The Laplace law is acceptable in such a situation. If a cardiac chamber of a heart patient increase its diameter to double, and a spherical chamber is assumed, it implies a two-fold greater fibre tension (Fig. 8-9B). To the patient, this means an enormous energy requirement in the myocardium to maintain the necessary pressure. This disorder is called cardiac failure or insufficiency (see later). Veins are highly distensible or compliant vessels (ie, they have a large volume/pressure ratio, dV/dP) that have one-way valves. The venous system (veins, venules, and venous sinuses) controls the amount of blood that is translocated from the venous to the arterial side of the circulation. In this paragraph the circulatory system is simplified to a venous system connected by a heart pump to an arterial system (Fig. 10-5A). The central venous pressure (CVP) is the pressure measured in the caval veins at the level of the heart or in the right atrium. When the heart pump stops, the pressure is the same in all compartments of the circulatory system, (ie, the mean circulatory equilibration pressure, MCEP). MCEP depends upon the blood volume and the compliance of the vessels (Fig. 10-5A). As the heart pump starts and moves blood from the venous system, the pressure here will fall and the arterial pressure will rise. A further rise in pumping activity (cardiac output) reduces the central venous pressure (CVP), and finally the CVP is negative, so the central vessels collapse. This impedes the bloodflow into the atrium (the venous return), so that the cardiac output cannot rise any longer (Fig. 10-5A). Fig. 10-5: The central venous pressure (CVP) as a function of cardiac output (A). – The cardiac output as a function of CVP (B). – Combined venous return and cardiac output curves as a function of CVP (C). The cardiac output must be a function of CVP at a given steady state. Increasing the venous return will increase CVP from –1 kPa towards zero, and increase cardiac output as well (Fig. 10-5B). As CVP becomes increasingly positive, it exerts a backpressure on the venous system to impede venous return. The rise in cardiac output levels off, and there is no further rise at values around MCEP. The third step is to combine the curves in Fig. 10-5 A and B. At the normal CVP (or right atrial pressure) the venous return curve crosses the cardiac output curve, and both flows are 5 l per min. If the atrial pressure is suddenly increased to the mean circulatory equilibration pressure, then all flow of blood is stopped (Fig. 10-5C). The low pressure during arrested circulation is mainly due to the very distensible venous system. The right atrial pressure has only increased slightly, but enough to decrease the venous return to zero and thus the cardiac output is zero (Fig. 10-5C). The more the atrial pressure falls below the venous pressure, the more the venous return will rise up to a certain level at an atrial pressure of -0.2 kPa (almost zero). Negative atrial pressures have the same venous return. This is because negative transmural pressures in the central, thoracic veins imply collapse. The venous return is therefore constant, regardless of a further fall in right atrial pressure (Fig. 10-5C). The cardiac output must equal venous return in the steady state. Thus the cardiac output- curve for the left ventricle must cross the venous return curve in one point (Fig. 10-5). The steep part of the cardiac output curve shows that the cardiac output can double following a small rise in pressure. The driving pressure for the systemic circulation is the mean aortic pressure (MAP) minus CVP. The relationship to cardiac output and total peripheral vascular resistance (TPVR) is given by Eq. 10-2. A small fraction of TPVR is found in the venous system. Eq 10-3 expresses the venous return. Small variations in CVP alter the volume of blood considerably in the venous system. A normal value for venule pressure is 1.3 kPa (10 mmHg) and for CVP about zero. Since venous return must equal cardiac output in steady state, the venous resistance is only about 10 % of TPVR. The venous pump is defined as all local external forces that facilitate venous return to the heart. Two important pump mechanisms are involved: 1. The skeletal muscle- pump. The deep veins of the arms and legs are affected by pressure exerted by exercising skeletal muscles. The veins are compressed by muscle contraction, and the one-way valves prevent the blood from flowing backward, and thus secure the transfer of blood toward the heart. Even the superficial veins are compressed during contraction. As soon as a venous segment is emptied of blood, its transmural pressure is so low that the filling pressure from more peripheral veins can fill the empty segment with blood. The skeletal muscular venous pump is also called the peripheral venous heart. In the erect position the peripheral venous heart must overcome the force of gravity and prevent overpressure in the dependent limb. During muscle rest there is an added hydrostatic pressure load of 13.3 kPa (100 mmHg) in the dependent limb. With a MAP of also 13.3 kPa the total pressure in a foot artery is 26.6 kPa, and in the dependent vein just above 13.3 kPa. During muscle contraction the venous pressure rises driving blood into the heart and just after muscle contraction the venous pressure falls again. 2. The thoraco-abdominal pump. The large veins are also affected by the positive intra-abdominal pressure and by the negative pressure in the thoracic cavity. The inferior caval vein returns blood from lower regions to the heart. During inspiration the intrathoracic pressure becomes more negative, and blood is sucked into the caval veins facilitating venous return to the heart. The inspiratory contraction of the diaphragm increases abdominal pressure favouring venous return. During expiration the intra-abdominal pressure decreases and intrathoracic pressure increases but remains negative, so that the venous return is maintained. Intrinsic cardiac mechanisms, including the length-tension relation (Chapter 2), allow the heart to increase stroke volume beat-by-beat, when the venous return increases. – Straining against a closed glottis is called Valsalva´s manoeuvre, and it is part of our every day life during coughing, defaecation, urination and lifting of heavy weights. The intrathoracic pressure increases abruptly, whereby the venous return is inhibited and the cardiac output is reduced (Starling´s law). Normally, fainting is prevented by a strong arteriolar and venous constriction released by the baroreceptor reflex. This paragraph is inserted here in order to help the reader understand the pathophysiology of the most common cardiovascular disorders. Lipoprotein particles are build up by a non-polar core containing triglycerides (TG) and cholesterol esters (E in Fig. 10-6). The polar shell of each particle consists of phospholipids, apoproteins and non-esterified cholesterol. These substances provide the particle with a negative electrical charge, which allow it to remain soluble in plasma (Fig. 10-6 right). Hepatic synthesis of cholesterol varies inversely with the dietary intake. Fig. 10-6: Lipoprotein metabolism (left). - The lipoprotein particles are found both in the plasma of healthy persons and of patients with atherosclerosis (right). Four different lipoprotein particles (VLDL, IDL, LDL and HDL) control the transport of cholesterol to the cells. a) VLDL, IDL and LDLVery low-density lipoproteins (VLDLs), which contain mainly the liver-produced TG, are synthesised in the liver and carries a characteristic surface protein called apoproteins B-100 (Fig. 10-6 right). VLDL is liberated from the liver in the postabsorptive phase Chylomicrons are formed in the enterocyte from dietary fat after a meal and absorbed from the intestine into the blood (Fig. 22-13). Cylomicrons contain all the dietary lipids including lipophilic vitamins and have a half-life of 5 min. VLDL from the liver, and chylomicrons absorbed from the intestine, are hydrolysed by the enzyme lipoprotein lipase (LPL) on the endothelial surfaces in the capillaries into glycerol, chylomicron remnants and free fatty acids (FFAs). From the FFAs the TG molecules are resynthesised and used or stored in adipocytes, heart and striated muscle cells (Fig. 10-6). As more and more TG is removed from VLDL, the density of the particles becomes greater, and they are now termed intermediate density lipoproteins (IDLs). Normally, the liver cells take up half of the IDL particles, because they have receptors for the apoprotein B-100 on the IDL surface. The hepatic lipase removes TG from the IDLs to produce low-density lipoproteins (LDLs) still maintaining their apoprotein B-100. This apoprotein is recognised by the LDL receptors of all cells. LDL is the largest cholesterol fraction in blood plasma, and has a half-life of 24 hours. LDL delivers cholesterol and other lipids from the liver to the cells for metabolic and structural purposes (forward transport). An increasing concentration of cholesterol inside the cell automatically down-regulates LDL receptors and thus regulates the receptor-mediated endocytosis of additional LDL. The circulating LDL concentration is controlled by the number of hepatic LDL receptors and by enzyme activity in the cholesterol synthetic pathway. Genetic LDL receptor deficiency elevates the ratio of LDL to HDL in blood plasma, and a ratio greater than 4 is a serious risk factor for cardiovascular disease. b) HDLThe remains of the chylomicrons co-operate with IDL and high-density lipoproteins (HDLs) to form cholesterol esters. Cholesterol esters are then exchanged for TG in VLDL and chylomicrons by the cholesterol ester-transfer protein, whereby HDL3 changes to the less dense HDL2. HDL is the substrate for lecithin-cholesterol acyltransferase (LCAT). This enzyme catalyses the conversion of free cholesterol to cholesterol ester. LCAT is reduced in severe liver disease. HDLs in plasma are disk formed particles, mainly produced in the liver. They contain an entirely different apoprotein called apoprotein Apo-I or Apo-II, and also Apo-E (Fig. 10-6 right). In the fasting state, the HDL concentration in the blood plasma is generally increased in females, by oestrogens, by exercise, and by moderate alcohol intake. Similarly, fasting HDL concentrations are reduced (and LDL increased) in males, by androgens, by smoking, by obesity, and by an inactive sedentary life-style. The cell membranes contain specific HDL receptors. HDL absorbs cholesterol in peripheral tissues and thereby matures from the nascent state (Fig. 10-6 right). LCAT is activated by the apoprotein A on the HDL surface. Mature HDL facilitates the transport of cholesterol back to the liver ((backward transport or reverse cholesterol uptake), where it binds to Apo-E. Normally, HDL particles carry 30% of the total quantity of cholesterol in the blood. HDL protects against development of atherosclerosis, and a high HDL/LDL ratio reduces the risk of cardiovascular disease. Population groups at risk are advised to eat a low fat diet with unsaturated lipids and a low cholesterol content, in order to prevent or delay the development of atherosclerosis. Persons with extremely low total cholesterol in their plasma demonstrate a higher mortality than persons with values around 5 mM. The reason for the increased mortality (mainly death of cancer and gastrointestinal diseases), is probably insufficient immune defence and genetic factors. A risk factor for a disease is a factor showing a statistical co-variance with the disease. A risk factor is not identical with a definite disease factor, where all causal steps are clearly understood. Nevertheless, risk factors may obtain an increasing degree of causal relationship to the disease. Two or more risk factors present frequently potentiates the risk. Major risk factors that predispose to atherosclerosis and ischaemic heart disease (IHD is atherosclerosis if the coronary arteries) are consequences of the Western World lifestyle. These consequences are often notified as age changes. The fact remains that the western life style is characterised by unhealthy fast-food, a high dietary fat fraction, obesity, years with lack of exercise, low fitness, smoking, hypertension, hyperlipidaemia, diabetes, gout, oral contraceptives (synthetic steroids), drugs and doping (testosterone or other steroids in excess). Consideration of risk factors must be supplied with other relevant information in order to provide a whole patient status, including genetic and immunological factors as mentioned above. The inactive lifestyle of the Western World is documented by measurement of a low maximal oxygen uptake (endurance capacity) as an average for large population groups. Values below 34 ml per kg and per min are unhealthy for any age. Such a low endurance capacity is related to high mortality (Chapter 18), especially from IHD (males and postmenopausal females). Regular exercise seems to protect against IHD. The following risk factors for IHD are dealt with below: Obesity, male sex, smoking, hyperlipidaemia, familial hypercholesterolaemia, diabetes mellitus, gout and asymptomatic hyperuricaemia, oral contraceptives or synthetic steroids for doping. Obesity is clearly associated with IHD, but probably not linked independently to IHD. The obese patient is characterised by an inactive lifestyle, low fitness, and preferring a fatty diet. Sex. Males are more frequently affected by coronary artery disease (IHD) than fertile females. The smaller incidence in females is obviously related to the presence of natural female sex hormones (oestradiol in natural dosage). After the menopause, the female incidence approaches that of males. Female sex hormones in natural dosage may be protective, and male sex hormones atherogenic. Testosterone stimulates hepatic cholesterol synthesis. Smoking a certain number of cigarettes per day is directly related to the incidence if IHD, and following 10 years of abstention, the risk declines towards the normal level. Hypertension is associated with an increased risk of IHD (Chapter 9). Most forms of hypotensive therapy only reduce the risk of cardiac events to some extent, but clearly reduce the risk of stroke. Some hypotensive drugs reduce the arterial blood pressure but still have unwanted side effects. Light exercise reduces the blood pressure and implies other beneficial effects. Hyperlipidaemia. Total cholesterol, HDL with calculation of LDL, and triglyceride concentrations should be measured in all patients. High total serum cholesterol combined with a low HDL, and also high triglycerides are associated with an increased risk of IHD (Fig. 10-6). Heterozygous familial hypercholesterolaemia is a relatively common genetic defect caused by mutations in the gene coding for the LDL receptor. With defective genes there is malproduction of LDL receptors in the liver. Some patients are without physical signs – others have cholesterol deposition around the eyes (xanthelasma) or in the tendons (xanthomas). These patients require diet with fibres and reduction of the cholesterol intake. Alcohol consumption must be reduced. The body weight must be kept close to ideal with exercise. Usually the patients require treatment with lipid-lowering drugs. Homozygous familial hypercholesterolaemia is extremely rare. These patients are without LDL receptors in the liver, so they accumulate cholesterol and other lipids in the aorta, arteries, organs and skin. The HDL/LDL ratio in blood plasma is greatly reduced (below 1:4), and the fasting total cholesterol increases towards 30 mM. Drugs are needed, but the patients usually die young from ischaemic heart disease. Diabetes mellitus. Increased blood glucose after fasting and an abnormal glucose tolerance test is associated with increased risk of atherosclerosis and a high LDL (Chapter 27). Gout and asymptomatic hyperuricaemia (Chapter 20) is associated with an increased risk of ischaemic heart disease and atherosclerosis. Intake of several types of oral contraceptives or synthetic steroids for doping (Chapter 18) increases the risk of atherosclerosis and thrombo-embolic phenomena. This paragraph deals with heart disorders and atherosclerosis, which cause most people of the Western Hemisphere to die. Coronary or Ischaemic Heart Disease (CHD or IHD) is the most widespread. The remaining cases are caused by arteriosclerotic damage of the brain (strokes) and other organs (liver, kidney etc). The two typical cardiovascular disorders are I. Atherosclerosis and II. Rheumatic heart disease. - Atherosclerosis is involved in several widespread disorders (ischaemic heart disease, peripheral arterial disease and hypertension). These diseases are related to the typical life style of the Western Hemisphere, whereas rheumatic heart disease is more frequent in poor countries with high frequency of infections and malnutrition. Atherosclerosis is a process of progressive lipid accumulation (atheromatosis) and calcification of the inner arterial walls in the abdominal aorta, lower extremities and the arteries of the heart, brain and kidneys. Atherosclerotic plaques contain cholesterol, and the most important single factor for their development is a high plasma concentration of total cholesterol, in particular a high concentration of LDL. Atheromas are yellow streaks or lesions found in arteries at autopsy. They are formed in the intima (lamina intima) by lipid accumulation in macrophages and monocyte adhesion. As more and more cholesterol crystals are deposited, the atheromas grow and the surrounding fibrous and smooth muscle tissue is involved. Finally – as the subendothelial distortion leads to platelet aggregation - large arteriosclerotic plaques are formed. They consist of cholesterol and other lipids, dead cells, collagenous fibres, and there is excessive proliferation of the smooth muscle cells. The fibrosis or sclerosis makes the arterial wall stiff, which lead to systolic hypertension. Later Ca2+ salts precipitate and a factual calcification of the arterial wall may occur. Typical for atherosclerosis patients are a high total cholesterol concentration in the blood plasma (total cholesterol above 6.2 mM), a dangerously high LDL and a low HDL fraction in fasting plasma (below 20% of the total). Often the atherosclerotic patient also has a high total triglyceride concentration (above 2 mM). In a fasted patient the triglyceride concentration depicts the precursor concentration of dangerous cholesterol: Very Low Density Lipoprotein (VLDL). Large atherosclerotic plaques narrow the arterial lumen and produce arterial stenosis with reduced bloodflow. Insufficient oxygen delivery to the tissue is called ischaemic hypoxia, and hypoxic pains develop as in angina pectoris and intermittent claudication. Total occlusion of the arterial lumen is caused by a thrombus or an embolus in the lumen, or by wall bleeding. Disruption of the endothelium results in accumulation of thrombocytes and fibrin with thrombus formation and a complex atheromatous lesion is produced. Arteriosclerosis (atherosclerosis) manifests itself in the coronary arteries as Ia. ischaemic heart disease and in peripheral arteries as Ib. peripheral arteriosclerosis. Ia. Ischaemic heart disease (IHD) Atherosclerotic coronary artery disease remains a leading cause of death, and is manifested as focal narrowing in the epicardial coronary arteries. The gradually narrowed vessel segment can be abruptly occluded by clot formation (thrombus) or by vasoconstriction at the atherosclerotic lesion. When a thrombus flows along the arterial tree with the blood and occludes the vessel, it is called an embolus. Ischaemic heart disease is caused by reduced bloodflow to a region of the myocardium. Myocardial ischaemia diminish delivery of oxygen and nutrients, and accumulate potentially toxic substances such as lactic acid and K+ around the cardiac cells, whereby necrosis may result. The causes are atherosclerosis with atheromas, thrombosis, emboli, or spasms in the coronary arteries. Fig. 10-7: Bloodflow through the left coronary artery at rest and during exercise in a healthy person (upper) and in a patient with coronary obstruction (lower) and angina pectoris. Coronary bloodflow is restricted in the systole by strong myocardial contractions and in diastole by the high heart rate of exercise. Normally, the coronary bloodflow in healthy persons is small during systole and increases during diastole (Fig. 10-7A). The high heart rate at exercise implies a short diastolic duration, but the rise in pressure secures a great diastolic bloodflow (Fig. 10-7B). Four clinical manifestations of IHD are treated below: 1) Angina pectoris (chest pains) is pain felt beneath the sternum or in the precordial area - often referred to the left arm-shoulder-neck-jaw etc. Exercise and cold bring on hypoxia pains in the substernal or precordial area. Hypoxic pains are transmitted by subendocardially situated nerve fibres. The coronary resistance vessels contain a-adrenergic constrictor receptors and badrenergic dilatator receptors. The vasodilatatory capacity of the coronary resistance vessels can be maximally mobilised already at rest (Fig. 10-7C). Exercise shortens the diastolic duration and restricts the rise in diastolic bloodflow further (Fig. 10-7D). The aggravated myocardial ischaemia results in a lactate acidosis. Following sublingual administration of nitro-glycerine, peak concentrations are achieved in the plasma within 1 min. Organic nitrates dilatate constricted coronary vessels, improve the bloodflow to the subendocardial (pain sensitive) part of the myocardium, and dilatate resistance & capacitance vessels. This dilatation reduces the venous return to the heart and the arterial pressure (reduced preload and afterload). The beneficial effect of drugs such as glycerol- trinitrate in angina have been known for more than a century. Recently it was realised that the drugs act by releasing nitric oxide (NO) in the vascular wall (see Chapter 5). Ca2+-channel blockers block the Ca2+ flux into the smooth muscle cells of the coronary arteries, so they relax. The Ca2+ -channel blockers also reduce the force of contraction and thus the oxygen demand of the myocardium. Coronary angioplasty is a method by which atheromatous obstructions are dilatated by an inflated balloon. The arterial oxygen concentration is also reduced in anaemia, CO poisoning and in shock. Patients with hyperthyroidism or hypertension may have increased coronary oxygen demand and all these paitients may experience chest pains caused by myocardial hypoxia. Another manifestation of ischaemic heart disease is myocardial infarction. 2) Acute myocardial infarction (AMI) is due to a sudden coronary thrombosis from an atheromatous plaque causing cellular death (infarct) of a myocardial area. Distal to the coronary occlusion the blood pressure is low. The thin-walled subendocardial vessels are squeezed most and receive the smallest bloodflow, often leading to subendocardial infarcts. The myocardial infarcts are sometimes silent (which means without pains; the pain relief is due to destruction of subendocardial nerve fibres). The typical infarct causes severe and long lasting pain. Acute myocardial infarction renders the heart incapable of pumping the minimal blood volume required to transfer sufficient oxygen to the mitochondria. The patient experiences a sudden chest pain and the pain is lasting for hours in contrast to angina. The patient may develop signs of shock. Necrotic myocardial cells liberate cellular enzymes such as creatine kinase, which peaks in the blood plasma within 24 hours. The total enzyme release depicts the size of the infarction. Lactic dehydrogenase (LDH) isoenzymes peaks a few days later, and LDH 1 is rather specific for myocardial necrosis. Read Chapter 11 before this paragraph: Non-Q wave infarction: When only part of the wall is necrotic there are deeply inverted, symmetrical T-waves (coronary T- waves) and mostly ST depression in the ECG. These signs of ischaemia are often transient - only during the acute attack - and found in all precordial leads located above the infarcted area. Such a subendocardial infarct does not show deep Q waves, and epicardial involvement implies ST segment elevation.
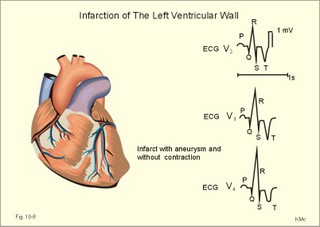
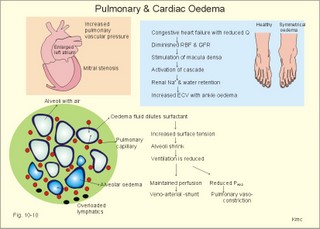
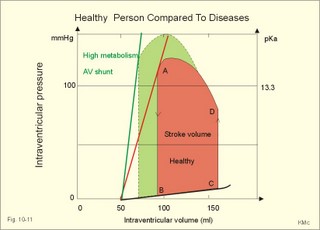
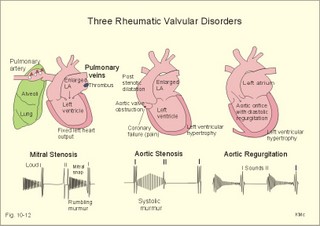
Fig. 10-8: Myocardial infarction of the left ventricular wall with lack of movement of infarcted tissue during systole. The ECG changes are typical for the anterolateral location of the infarct (see Ch. 11). Q-wave infarction: A wide and deep Q wave in the ECG is a lesion wave, and the sign of transmural myocardial infarction with necrosis through the whole of the myocardial wall. The deep Q wave is maintained for years after the event. A typical anterior wall infarction shows changes in lead I and in V2-V6 dependent upon the localisation (Fig. 10-8). During systole the infarcted area does not contract or move due to cell necrosis (paradoxical movement). There is always a danger of rupture of necrotic tissue. A typical posterior wall infarction is diagnosed by a mirror image with changes in V1-V2 (reciprocal changes) and ST- depression in lead I. ST-depression or ST-elevation is indicative of myocardial ischaemia. Chapter 11 is a prerequisite for the understanding of the above paragraph! Fig. 10-9: Left ventricular pressure-volume loops in a healthy person (red) and for persons with acute (blue area) or chronic, congestive (green area) cardiac failure. The AMI patient is extremely tired. Even the work of breathing is a heavy task. The condition is often a case of general ischaemic hypoxia (Fig. 10-9) and can develop into cardiogenic shock (see below). Interaction of platelets with collagen in the vessel wall is the first step in platelet aggregation leading to thrombosis. The activated platelets release thromboxanes A2 (TxA2 ) from arachidonic acid in the phospholipids of the platelet membrane. Platelet aggregation is inhibited by cAMP and by acetyl-salicylic acid, which inhibits platelet cyclo-oxygenase. Eicosapentaenoic acid (EPA) in the diet reduces the frequency of thrombosis by reduction of the TxA2 production. 3) Cardiac failure or cardiac insufficiency is a disorder, where the heart cannot pump enough blood to satisfy the nutritive needs of the body. Cardiac insufficiency is manifest by a consequential decrease in cardiac output (lower output failure) or by an increase in cardiac output (higher output failure). The cardiac failure can be acute or chronic. Damming of blood in the vessels behind the insufficient heart pump is typical. Acute cardiac failure is caused by AMI, acute intoxications, anaesthesia etc. Occlusion of the coronary artery to the left ventricle impairs contractility, and left ventricular failure develops due to the diminished cardiac output from the left ventricle. Initially, the right ventricular output is maintained, whereby the left atrial pressure (and pulmonary venous pressure) is increased beat-by-beat. As a consequence, the left ventricular output will increase until the cardiac outputs of the two ventricles are equal. The increased pulmonary venous pressure leads to reduced lung compliance (dV/dP) and increased respiratory elastic resistance with increased respiratory work and distress. Eventually, plasma fluid flows into the alveoli and pulmonary oedema is developed (Fig. 10-10). Chronic or congestive cardiac failure occurs in conditions such as IHD and following severe hypertension. In chronic cardiac failure blood is accumulated and expands the venous system and the left ventricle (Fig. 10-9). Cardiac oedema develops during congestive cardiac failure, because the kidneys retain NaCl and water. The accumulated fluid increases venous return, which in turn elevates the right atrial pressure. The rising atrial pressure elevates the venous and the capillary pressure. This causes loss of fluid into the interstitial fluid volume. Accumulation of abnormal volumes of interstitial fluid is the definition of oedema. The low cardiac output and blood pressure causes an increased sympathetic tone with constriction of the afferent renal arterioles to the glomeruli. As a consequence, the renal bloodflow (RBF), and the glomerular filtration rate (GFR) decrease (Fig. 10-10). Also the NaCl concentration decreases in the renal macula densa (Fig. 25-17). The renin-angiotensin-aldosterone cascade is activated, which enhances salt-and water-retention. Angiotensin II is a strong vasoconstrictor, which further decreases the renal bloodflow, and aldosterone promotes the reabsorption of NaCl and water from the distal renal system. A certain salt-water retention is beneficial in the early stages of cardiac failure, because of improved venous return and thus improved cardiac output according to Starlings law of the heart. However, prolonged activation of the renin-angiotensin-aldosterone cascade and the sympathetic nervous system, damage the heart muscle further and reduce its contractility. This is because circulating vasoconstrictors, such as catecholamines, vasopressin and angiotensin II, imply an extra workload on the damaged myocardium. When the salt and water retention results in even a small rise in the osmolarity of plasma, there is a stimulus of osmoreceptors, located close to the neurosecretory cells in the hypothalamus. The osmoreceptors stimulate both production and secretion of vasopressin (antidiuretic hormone, ADH) in the neurosecretory cells. ADH eases the renal reabsorption of water in the outer cortical collecting ducts leading to a low urine flow (antidiuresis). Vasopressin is also an universal vasoconstrictor. Fig. 10-10: Formation of pulmonary oedema in left ventricular failure (mitral stenosis) and in congestive cardiac failure with ankle oedema. Increased venous pressure with stasis of blood dilatates the central vessels and the heart chambers. The distended atrial wall liberates atrial natriuretic peptide (ANP), which increases Na+-excretion and dilatates peripheral vessels (Chapter 24). This is a partial compensation of the increased preload (the water loss by high urine flow reduces venous return) and afterload (the vasodilatation reduces outflow resistance). Venoconstriction shifts significant quantities of blood from the peripheral to the central circulation. Since central venous pressure (CVP) varies inversely with TPVR, it is possible to maintain cardiac output in resting patients with congestive heart failure (insufficient contractile force) at the expense of increased CVP, by reduction of TPVR (Eq. 10-2). When cardiac output decreases more and more during development of congestive heart failure, the compensation fails, and both CVP and end-diastolic ventricular pressure (preload) and volume rises further (Fig. 10-9). The superficial meck veins are expanded, when CVP is abnormally elevated. Eventually, large volumes of plasma water flow from the liver into the peritoneal cavity due to the elevated CVP. Fluid accumulation in the abdomen is called ascites. Patients with a cardiac output much higher than normal can develop cardiac failure. The venous return is much too high, and after some time with an overexpansion of the heart, the cardiac pump fails to eject the same blood volume as it receives, and an increasing blood volume is accumulated behind the insufficient ventricle. The rise in left atrial pressure leads to pulmonary oedema and eventually the right ventricle fails so peripheral oedema develops. Examples of this condition are cardiovascular disorders with a drastic reduction of the TPVR. The low opening pressure in the left ventricle being equal to a low end-diastolic aortic pressure (Fig. 10-11) illustrates the low TPVR. Fig. 10-11: Left ventricular pressure-volume loops from a healthy person, and from a person with high metabolic rate (hyperthyroidism) - or a person with arteriovenous shunts. In hyperthyroidism (a disease with an abnormally high metabolic rate described in Chapter 28), all the vessels in the systemic circulation dilatate, and the venous return overloads the heart. On the other hand, short-term administration of L-thyroxin to patients with chronic heart failure improves cardiac and exercise performance. Any major arteriovenous shunt leads a large fraction of the arterial blood directly into the veins. This greatly increases venous return and overloads the heart (Fig. 10-11). 4) Cardiogenic shock - terminal pump failure - is such a severe reduction of cardiac output that the peripheral tissues suffer seriously from lack of oxygen, the cells deteriorate and within hours or days the patient die. The pulmonary capillary wedge pressure is normal or elevated in contrast to other types of shock (blood loss or vasodilatation). During insufficient pumping capacity and cardiac arrest, the cardiac pump do not get rid of the blood volume received and a large blood volume is therefore accumulated in the distensible venous system, pulmonary system and the thin-walled chambers of the heart. This is why the lower part of a newly diseased body is filled with blood in distensible vessels (livores), and the upper part of the body is pale. I b. Peripheral arteriosclerosis Peripheral arteriosclerosis refers to atherosclerosis and other changes of the large and medium large peripheral arteries. The muscular lamina media grows and becomes fibrotic often with atheromas in small arteries. The walls of the elastic arteries become thick of hyaline and the lumen narrows, which causes systolic hypertension. The narrowing also leads to ischaemia, which further promotes systolic hypertension. The most frequent of these disorders is chronic ischaemia of the legs, also called intermittent claudication from its prominent symptom. Claudication is a cramp-like pain, which occurs during exercise and subsides at rest. Occlusive atheromatous lesions between the common iliac and the common femoral artery lead to claudication of the thigh and calf. Lower femoral artery disease usually causes claudication of the calf, and occlusive lesions of the popliteal artery causes claudication of the calf or the foot. If possible, regular relaxed exercise should be undertaken in an attempt to develop anastomoses. In severe ischaemia there is pain at rest. Balloon dilatation is often useful, and amputation may become necessary. Rheumatic fever is caused by repeated pharyngeal infections with group A streptococcus. An autoimmune reaction is triggered by the streptococci and the patient develops fever, joint pains, diastolic mitral murmur caused by mitral valve inflammation, cardiac enlargement with pericardial effusion and pericarditis (raised ST-segment in ECG – see Chapter 11) or myocarditis (inverted T-waves). More than 50% of those who suffer from acute rheumatic fever with carditis develop rheumatic heart disease many years later. The rheumatic valvular disease mainly affects the mitral and the aortic valves. Mitral stenosis and regurgitation Practically all cases of mitral stenosis are caused by rheumatic heart disease. Severe mitral valve stenosis is present, when the mitral valve orifice is reduced to 10-4 m2 as compared to the normal area of (5 *10-4 m2). The left atrium dilatates and its walls hypertrophy in order to maintain sufficient bloodflow to the left ventricle. Obviously, the pressures also increase in the entire pulmonary vascular bed: veins, capillaries, arteries and right ventricle. The stenotic mitral valve and the resistance of the pulmonary arteries determine the pressure in the pulmonary capillary bed. Up to a certain point, pulmonary arteriolar vasoconstriction protects the patient from pulmonary oedema. Patients with severe mitral stenosis have cyanotic cheeks and ears (mitral faces) caused by stasis of the blood. Auscultation at the apex, lying on the left side just following exercise reveals a split second heart sound with a mitral snap (second component) as the mitral valves open, then a mid-diastolic rumbling murmur - like a sack of potatoes falling on a floor - caused by turbulence through the narrowed orifice. The murmur ends in a loud first heart sound, because the cusps are kept open until the start of the ventricular systole (Fig. 10-12). As the left atrium grows it becomes activated later than the right, and the P-wave is bifid (P- mitral). The large left atrium favours the development of atrial fibrillation with thrombo-embolism and emboli to the brain, kidneys and gastrointestinal tract. Mitral stenosis is often combined with regurgitation. Regurgitation is recognised by a systolic murmur without any 1.heart sound. The 1.heart sound is caused by the closure of the cusps, and in mitral stenosis they do not close. Usually the condition is asymptomatic for decades following rheumatic fever. Light pulmonary oedema presents itself as coughing and exertion dyspnoea. Replacement of the mitral valve is performed with artificial valves, which may work for decades with adequate anticoagulant therapy. Fig. 10-12: Mitral stenosis, aortic stenosis and aortic regurgitation. Aortic stenosis and regurgitation Disorders of the normal tricuspid aortic valve are mainly caused by rheumatic fever or by atherosclerosis. Almost half of all cases of rheumatic heart disease include the aortic orifice, usually associated with the mitral orifice. Symptoms and signs are characteristic: Exercise-induced syncope and angina. This occurs when the disease is severe and the area of the aortic orifice is reduced to 1/3 of normal. The left ventricular pressure rises and the left ventricle hypertrophies. The increased oxygen demand of the myocardium leads to ischaemia with angina pectoris, arrhythmias and left ventricular failure. Healthy persons can increase their cardiac output by a factor of 5 or more, but this is not possible for patients with severe aortic stenosis. The arterial blood pressure falls, the patient is pale (aortic face), chest pains worsen, and the patient may lose consciousness. There is a strong systolic murmur in the aortic area and over the carotid arteries. The echocardiogram demonstrates thick aortic valve cusps and left ventricular hypertrophy. A ventricular-aortic pressure gradient above 6.7 kPa (50 mmHg) measured by cardiac catheterisation, is indicative of surgery. Without surgical intervention death frequently ensues within a few years from the occurrence of the first serious signs. Tricuspid stenosis and regurgitation This is an uncommon complication, which is related to rheumatic heart disease or is congenital. Regurgitation is more frequent than stenosis, but often the two conditions are combined. Isolated tricuspid stenosis dilatates the right atrium and the caval veins, and the liver swell just like the condition with constriction of the heart. Atrial fibrillation is frequent from the dilatated chamber. Tricuspid regurgitation is a condition where the right ventricle delivers blood to both the pulmonary artery and the right atrium at each systole. The right heart is dilatated, whereas the lung vessels are not. Pulsation of the neck veins and a large tender liver are typical signs. There is often a blowing systolic murmur over the sternum. Replacement of the tricuspid valve is performed with artificial valves. Left ventricular hypertrophy is an abnormal increase of the left ventricular mass caused by increased demands of cardiac work. If due to pressure overload it is called concentric hypertrophy in which new contractile elements are lined up in parallel, and if due to volume overload it is called eccentric hypertrophy, in which new contractile elements elongate the myocardial cell. - This disease is described in Chapter 11. Other cardiovascular disorders Thrombo-angiitis obliterans (Buergers disease). Buerger´s disease occurs in small arteries of the limbs of young smokers – typically males. The vessel wall is inflamed, but many lesions look like atherosclerosis. The patient is invalid by intermittent claudication, and the only choice of treatment is to stop smoking. Raynaud´s disease is a condition with cold precipitated attacks of spasms of the small arteries and arterioles, supplying the fingers and toes. The disease is usually bilateral and affects predominantly young girls and female smokers. First the skin becomes pale and white from vasoconstriction, due to slow bloodflow, and finally red because of hyperaemia. The vasoconstriction occurs in the digital arteries, arterioles and skin capillaries. A few minutes later the capillary smooth muscle spasm is released due to local vasodilatators, and the capillaries are filled with oxygen poor blood (the skin becomes blue and is still cold). Finally, the arterial and arteriolar constriction is released and the classical physiological reactive hyperaemia occurs, with red, warm fingers and paresthesia (numbness). Centrally controlled vasoconstrictor tone, sensitive to cold signals, is probably implicated in cases with symmetrical spasms. A centrally increased vasoconstrictor tone involving the coronary bloodflow is consistent with the fact that some of the Raynaud patients also suffer from chest pains (angina pectoris) and migraine. Raynaud´s disease occurs in a primary and a secondary form. Primary Raynaud´s disease is a condition where the cause is unknown (ie essential). There is a benign familiar occurrence of so-called dead fingers (ie, digiti mortui familiaris), and a malignant form with symmetrical gangrene (ie, symmetric gangrene). Secondary Raynaud´s disease (Raynaud´s phenomenon) occurs together with connective tissue disorders (dematomyositis, polymyositis, systemic lupus erythematosus and systemic sclerosis). Raynaud´s phenomenon is also a side effect to treatment with b-blockers, in which case they must be withdrawn. The patient has to wear warm clothes in order to protect both the shell and the core temperature. Nifedipine is sometimes beneficial. Varicose veins have incomplete valves. Normally, the muscular venous pump maintains the venous bloodflow towards the heart. Patients with defective valves can develop venous pooling or stasis and ankle oedema. This is because the contracting leg muscles squeeze the blood in the retrograde as well as in the anterograde direction. Ventricular stroke work rate is the sum of the pressure-volume work and the kinetic work: Eq. 10-1: Stroke work rate = [(P × V) + ½ m * v2]. Both the pressure-volume work and the kinetic work are work per stroke duration or time unit that is comparable to work-rate or effect in Watts. The driving pressure for the systemic circulation is the mean aortic pressure (MAP) minus CVP. The relationship to cardiac output and total peripheral vascular resistance (TPVR) is given by: Eq. 10-2: Cardiac output = (MAP - CVP)/TPVR. A small fraction of TPVR is found in the venous system. The venous return is expressed by the approximative equation: Eq. 10-3: Venous return = (venule pressure - CVP)/ venous resistance. I. The following five statements have True/False options:
II. The following five statements have True/False options:
Three years following heart-lung transplantation, a patient is examined at the hospital for the cause of frequent exertion syncope. Heart catheterisation reveals that the systolic/diastolic pressure in the left ventricle is 26.7/0 kPa (200/0 mmHg), and in the aorta 10.7/6.7 kPa (80/50 mmHg). 1. What is the cause of exertion syncope? 2. What is a likely diagnosis? 3. Argue for the size of the left ventricular cavity and wall thickness. A 24-year old sporty male consults the doctor because of syncope while playing handball. The examination reveals a systolic murmur to the right in the second intercostal space aortic site). The systolic murmur is audible also over the neck. The arterial blood pressure is 145/85 mmHg (19.3/11.3 kPa). There is a history of rheumatic fever at the age of 11. ECG shows a deep S-wave in V1 and a high R-wave in V6 (the sum is 5 mV), and there are asymmetrically negative T-waves in V4-V6. 1. What is the most likely diagnosis? 2. What is the ECG diagnosis 3. Describe the prognosis and the therapy. A 55-year old female complains of headache and an arterial blood pressure of 190/100 mm Hg (25.3/13.3 kPa) is found. Her ECG shows deep negative S-waves in V1 and V2, and high R-waves in V5 and V6. The sum of one S- and one R-wave is above 4 mV. 1. Calculate the mean arterial pressure and compare the result to a normal value. 2. What is the diagnosis? 3. Is the patient suffering from any cardiac disease? 4. Why is the arterial pressure amplitude (eg, systolic minus diastolic pressure) much larger than normal? A girl, 17 years of age, with frequent episodes of acute tonsillitis and articular pain during her childhood, developed a cardiac disease. The diagnosis was made as the doctor heard a diastolic murmur over the precordial area. The main complaint of the girl was dyspnoea at exertion. Cardiac catheterisation revealed a mean pressure in the pulmonary artery of 58 mmHg (7.7 kPa), and in the left atrium 28 mmHg (3.7 kPa), whereas the pressure in the left ventricle was only 2 mmHg (0.27 kPa) in early diastole. The pressure in the aorta was 105/80 mmHg (14/10.6 kPa). The cardiac output was measured at rest with the Fick principle to be 2.5 l min-1. Her circulating blood volume is somewhat less than her total blood volume, and exactly 5000 ml. The blood volume of the systemic capillaries is 3% of 5000 ml.
A female, 33 years old, complains of attacks of severe pain in the fingers of both hands, when she is outdoors in cold weather. She is a heavy smoker since the age of 16. The patient also suffers from another pain disorder. She has half-sided headache with visual disturbances at least twice a month in the cold season. The patient explains the pain attacks in the fingers as follows. First the skin of both hands and the fingers (not the thumb) becomes pale and white, and they feel like dead. A few minutes later the skin is blue, and the pain is severe. After 5-10 minutes the skin suddenly becomes red and it is very painful. 1. What is the diagnosis of the finger disease? 2. Are the finger disease and the headache related? 3. Describe the pathophysiology of the finger disease. Try to solve the problems before looking up the answers. · The ventricular stroke work is the sum of the pressure-volume work and the kinetic work. · A good index of the afterload is the peak aortic pressure during systole. · A ventricular-aortic pressure gradient above 6.7 kPa (50 mmHg) is indicative of surgery in aortic stenosis. · The venous pump is defined as all local external forces that facilitate venous return to the heart. · The skeletal muscular venous pump is also called the peripheral venous heart, because its force must be equal to or larger than that of the heart in order to return the blood in the upright position. · During inspiration, the intrathoracic pressure becomes more negative, and blood is sucked into the large thoracic veins facilitating venous return to the heart. · Atherosclerosis is a process of progressive lipid accumulation (atheromatosis) and calcification of the inner arterial walls in the abdominal aorta, lower extremities and the arteries of the heart, brain and kidneys. · High-density lipoproteins (HDLs) in plasma are disk formed particles, mainly produced in the liver for cholesterol transport. HDLs protects against the development of atherosclerosis as they transport the cholesterol back to the liver, where the elimination begins. · Acute myocardial infarction renders the heart incapable of pumping the minimal blood volume required to transfer sufficient oxygen to the mitochondria at rest. · Cardiogenic shock - or terminal pump failure - is such a severe reduction of cardiac output that the peripheral tissues suffer seriously from lack of oxygen, the cells deteriorate and within hours or days the patient die. The pulmonary capillary wedge pressure is normal or elevated in contrast to other types of shock. · Left ventricular hypertrophy is an abnormal increase of the left ventricular mass caused by increased demands of cardiac work. If due to pressure overload it is called concentric hypertrophy in which new contractile elements are lined up in parallel, and if due to volume overload it is called eccentric hypertrophy, in which new contractile elements elongate the myocardial cell. · Cardiac oedema develops during chronic cardiac failure, because the kidneys retain fluid. · Buerger´s disease occurs in small arteries of the lower limbs of young male smokers. The vessel wall is inflamed, but many lesions look like atherosclerosis. The patient is invalid by intermittent claudication, and the only choice of treatment is to stop smoking. · Raynaud´s disease is a condition with cold precipitated attacks of spasms of the small arteries and arterioles, supplying the fingers and toes. The disease is usually bilateral and affects predominantly young girls and female smokers. · Rheumatic fever is caused by repeated pharyngeal infections with group A streptococcus. The streptococci trigger an autoimmune reaction and the patient develops fever, joint pains, diastolic mitral murmur caused by mitral valvulitis, cardiac enlargement with pericardial effusion and pericarditis or myocarditis. · More than 50% of those who suffer from acute rheumatic fever with carditis develop rheumatic heart disease many years later. · The rheumatic valvular disease mainly affects the mitral and the aortic valves. · Practically all cases of mitral stenosis are caused by rheumatic heart disease. Severe mitral valve stenosis is present, when the mitral valve orifice is reduced to 10-4 m2 as compared to the normal area of (5 *10-4 m2). · Disorders of the normal tricuspid aortic valve are mainly caused by rheumatic fever or by atherosclerosis. Almost half of all cases of rheumatic heart disease include the aortic orifice, usually associated with the mitral orifice. Von Spiegel T, G Wietash and A Hoeft (1998). Basics of myocardial pump function. Thorac Cardiovasc Surgery 46, Suppl 2, 237-41.
|
||
Click here to introduce your comments or contributions