New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 20: Metabolism & Nutritional Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 20
|
 |
|||||||||||||||||||||||||||||||
|
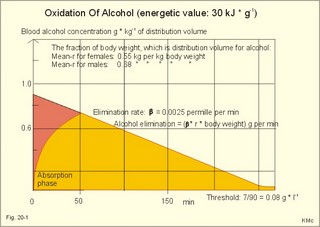
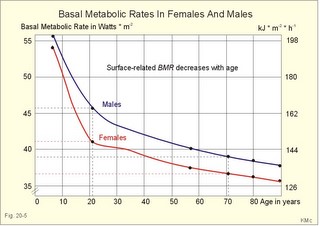
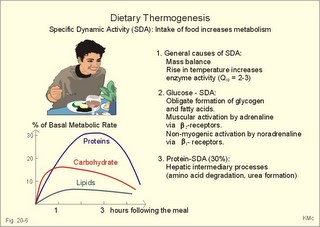
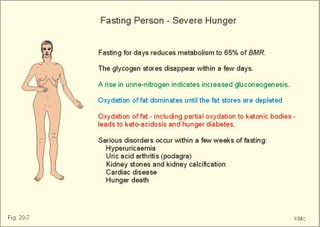
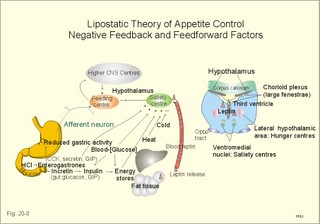
· To define heat energy, basal metabolic rate, Gibbs energy for ATP-formation, mechanical efficiency, metabolic rate, and the energy equivalent for oxygen. · To describe direct and indirect calorimetry, factors influencing metabolic rate and basal metabolic rate, and conditions with unsteady respiratory state. · To draw a curve for the combustion rate of alcohol. · To calculate a metabolic variable from relevant variables given. · To explain the alcohol metabolism and toxicity. To explain the control of appetite, dietary thermogenesis, energy balance, net combustion and RQ relations. To explain the first law of thermodynamics applied to humans. · To use the above concepts in problem solving and case histories. · The first law of thermodynamics. The internal energy of a system can change for any transition between two equilibrium states and is equal to the heat exchanged by the system and the work done by or on the system. – As a consequence, the metabolic heat energy transfer equals the heat loss plus the stored heat energy. · The surface law: The basal metabolic rate (BMR) per body surface area is much more uniform than the BMR per kg of body weight in individuals of the same species but of different form and size. The best expression for comparison is the BMR per kg of lean body mass. The lean body mass is the fat free mass. · Van´t Hoof´s rule: The rate of energy conversion in chemical reactions increases in proportion to the rise in temperature. · Basal metabolic rate (BMR) is defined as the metabolic rate measured with the subject awake in the morning, fasting, at neutral ambient temperature and resting horizontally in the respiratory steady state. · Body mass index (BMI) is the weight of the person in kg divided by the height (in m) squared. The normal range is 19-25 kg per square metre. · Brocas index is the predicted body weight in kg, which equals the height of the person in cm minus 100 for males and 110 for females. · Dietary thermogenesis is the increase in metabolic rate following food intake. · Energy balance is a condition, where the energy input equals the energy output, so the energy stores of the body are unchanged. · Gibbs energy is the free chemical energy in food, which is available for life. · Heat energy is energy transfer caused by a temperature gradient. · Ideal weight refers to the weight associated with the highest statistical life expectancy. The ideal weight is determined with the Brocas index or with prediction tables. · Inactivity is defined as a low endurance capacity (ie, a maximal oxygen uptake below 34 ml * min-1*kg-1). Inactivity is probably involved in development of life-style risk factors. · Lean body mass is the fat free body mass. · Marasmus is the result of universal starvation in a child, who has low body weight, muscle wasting, and the look of an old person. · Mechanical efficiency is the ratio between external work and the total energy used during work. · Metabolism is defined as the sum of all chemical processes in which energy is made available and consumed in the body. · Metabolic rate (MR) is defined as the decrease in internal energy (enthalpy) of the body in a given time period. The metabolic rate refers to the measurement in energy units with direct or indirect calorimetry. · Net metabolism is the stochiometric sum of the net reactions in the body. · Nitrogen balance is a condition, where the nitrogen input from absorbed amino acids equals the nitrogen output in the urine. · Obesity implies the excess storage of fat, and is defined as an actual body weight exceeding the ideal weight by more than 20%, or by a body mass index above 30 kg per square metre (WHO). · Protein deficiency (kwashiorkor) is starvation in children, which subsists on a protein-poor diet rich in carbohydrates. · Respiratory Quotient (RQ) and ventilatory exchange ratio (R) is defined in Chapter 14. · Teratogens refer to all chemical, physical and biological agents that cause developmental abnormalities (teraton means monster). · Vitamins are essential organic catalysts in the diet, necessary for normal metabolic functions in humans, but not synthesized in the human body. · Respiratory steady state is a condition where RQ equals R. This paragraph deals with 1. Energy exchange, 2. Metabolism, 3. Alcohol, 4. The respiratory quotient and R, 5. Net mechanical efficiency, 6. Energy sources, 7. Direct calorimetry, 8. Native diets, and 9. Control of energy balance. It is generally believed that nutrients are necessary in order to produce energy in the human body. However, this is impossible. The first law of thermodynamics states that energy can neither be created nor destroyed but is transferred from one form to another or from one place to another. Life is thermodynamically the maintenance of an infinite row of non-equilibrium reactions in such a way that appear to be in a stationary condition, a steady state. Real life is chaos, a steady state only maintained as long as we derive chemical energy from food. Only part of the dietary energy is available for ATP formation in humans. Cellulose, for example, passes the digestive tract without being absorbed. The absorbable chemical energy passes through the intestinal mucosa, and is in the body transformed to energy rich phosphate bindings in ATP (Gibbs-energy, DG). ATP is broken down to ADP during muscular contractions. Muscular contractions stimulate the oxidation of fatty acids and carbohydrates in the muscle cells which liberate more energy for rephosphorylation of ADP to ATP. The energy is used for the maintenance of chemical syntheses, electrochemical potentials and for the net-transport of substances across membranes. The Gibbs energy is the free chemical energy available in food. However, 75% is lost as heat energy, and the mechanical efficiency of exercise is therefore only 25%. The ratio between external work (W') and the total energy used during work (-DU) is called the mechanical efficiency. In this case DU equals DG. The mechanical efficiency is always less than one and often only 0.25 as stated above. The energy, which is not transferred to external work, is released as heat energy (-Q) or is accumulated in the body as heat. At the onset of exercise 50% of the total energy from hydrolysis of ATP is converted into mechanical energy in the myofibrils. The remaining 50% are lost as initial heat. As shown above the mechanical efficiency is only 25%, however. This is because energy recapturing recovery processes (oxidative regeneration of ATP etc) occur outside the myofibrils. Hereby, half of the energy is dissipated as so-called recovery heat. Heat energy is low prize energy. In contrast to ATP energy, it is not available for work in the body. The sum of heat energy generated and work performed is constant and equal to the Gibbs energy. When no work is performed W' is zero, and all body reactions are reflected by the liberated heat energy (-Q), which is equal to the decrease in Gibbs energy (-DG). When the pressure-volume work is zero, we have a special energy concept: the heat content or enthalpy, H, which sums up all energy. The sum of liberated heat energy (Q) and liberated work (-W') is thus equal to the fall in enthalpy (Eq. 20-1). The decrease in enthalpy of the human body (-DH) is equal to the fall in potential, chemical energy stored in the body. The decrease in Gibbs energy covers almost the total energy, except for the pressure-volume work. Since oxygen consumption is almost equal to the carbon dioxide output, the pulmonary volume change is negligible and this work is negligible. The metabolism of a person is defined as the sum of all chemical reactions in which energy is made available and consumed in the body. The bindings between hydrogen and carbon in nutrients are a source of energy for animals. Such substances are changed into metabolic end products (eliminated as bilirubin, urobilin, urea, uric acid, creatinine etc.) and to metabolic intermediary products (ie, products that participate in other chemical reactions). The net metabolism is the sum stochiometry of the single net reactions in the body. The decrease in enthalpy in a given time period (-DH/min) is the metabolic rate (MR). The oxidation of fuel (carbohydrates, glycerol, fatty acids) to CO2 and water is the primary pathway for generation of energy and subsequent heat energy liberation. Protein can also serve as an important energy source during prolonged exercise, but it must first be broken down to amino acids, who are then partially oxidised (to CO2, water, NH4+ etc). The daily production of metabolic water is 350 g and of urea 30 g. Diabetes mellitus and hunger (hunger diabetes) are conditions where fatty acids can produce ketone bodies. During forceful exercise, energy is obtained primarily from non-oxidative sources (glycolysis). There is, therefore, a net formation of lactic acid from glycogen. Following anaerobic exercise the lactate elimination accounts for an extra O2 consumption called oxygen debt (Chapter 18). Oxidation of alcohol can contribute to metabolism. The energetic value of alcohol is 30 kJ/g. An adult person of 70 kg body weight can combust 7 g of alcohol per hour (see calculation below). The chemical energy liberated is (7 × 30) = 210 kJ per hour or 70% of his resting MR (300 kJ per hour or 83 Watts). Most of the chemical reactions in our body are degradative or catabolic - they break a molecule down to smaller units. These reactions are often also exothermic (heat releasing) and exergonic (the content of Gibbs energy decreases during these reactions). The synthetic or anabolic reactions (the formation of protein from amino acids) are obviously coupled to these degradative reactions. Synthetic reactions are most often also endothermic and endergonic. Alcohol diffuses easily in the human body. 20% of the intake by drinking is already absorbed in the stomach. The absorption is fast and is stimulated by CO2 (champagne). Alcohol distributes in the total water of the body within one hour. The distribution volume depends upon the fat mass, because fat tissue only contains 10% of water. The Swedish scientist Widmark called the fraction of the body weight, which is distribution volume for alcohol, r. The values for r varies considerably, but the mean-r for females is 0.55 and for males it is 0.68 kg per kg of body weight. The blood alcohol concentration is measured in permille (ie, one g of alcohol per kg of distribution volume). The most important elimination of alcohol is by oxidation. The rate of alcohol oxidation is constant (b = 0.0025 permille per min) and is independent of the blood alcohol concentration. The absolute amount of alcohol eliminated per minute is: (b × r × body weight) - see Eq. 20-4. The constant rate is due to the primary, partial oxidation to acetate via acetaldehyde in the liver by alcohol dehydrogenase: C2H5OH + 02 « CH3COOH + H20. Acetate is broken down in nearly all tissues. The total oxidation of alcohol: C2H5OH + 3 02 Þ 2 CO2 + 3 H20 implies an RQ of 2/3. A healthy person with a metabolic rate (MR) of one mol O2 per hour can partially oxidise almost 1/6 mol of alcohol per hour, by using almost 1/6 of his MR in the liver (one mol = 46 g alcohol; 46/6 or about 7 g alcohol per hour). Fig. 20-1: Absorption and oxidation of alcohol. If this standard person receives an alcohol infusion of 7 g per hour and has a normal hepatic bloodflow of 90 l per hour (1.5 × 60 min), his maximal alcohol elimination rate corresponds to a blood [alcohol] of (7/90) = 0.08 g per l. This is a blood [alcohol] threshold below, which the oxidation rate decreases with time (Fig. 20-1). The excretion of alcohol molecules takes place through expiratory air, urine and sweat. This excretion is generally considered to be negligible compared to the oxidation. This is actually true at rest (Table 20-1). A resting athlete with a blood [alcohol] of one permille or 1 g per kg has a small alcohol partial fraction in pulmonary blood and alveolar air (1/2000- 1/2100). With an alveolar ventilation of 5 l BTPS per min at rest, this person excretes 0.15 g each hour via expiratory air (Table 20-1). The concentration of alcohol in plasma water, sweat and urine is 20% (1.2 fold) higher than the blood [alcohol]. A resting athlete with a diuresis of one ml/min or 0.060 l per hour excretes alcohol by renal ultrafiltration at a rate of only 0.072-g per hour. If the person also has a sweat loss of 0.1 l each hour, he further excretes 0.12 g per hour. The total excretion at rest is only 0.342 g each hour (Table 20-1).
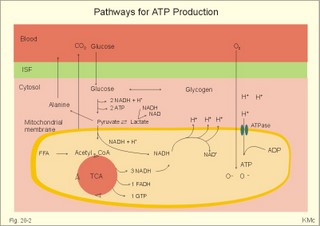
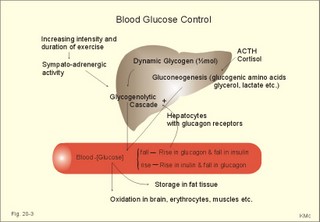
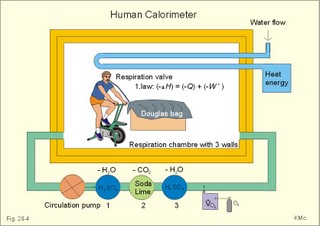
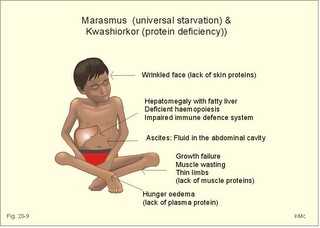
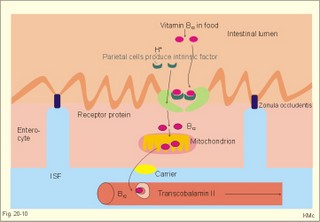
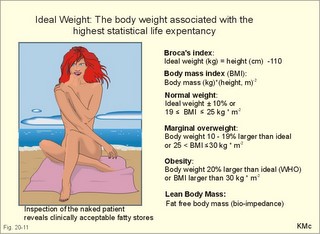
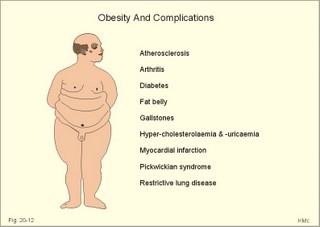
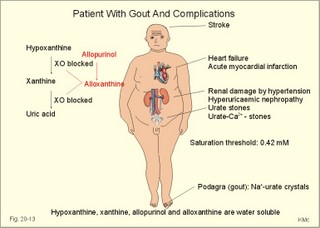
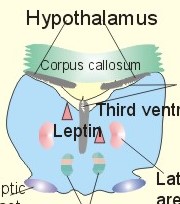
However, during one hour of exercise in a warm climate, when ventilation is 80 l per min, and when water loss is 4 l per hour (sweat and evaporation), the total alcohol excretion of the athlete is 7.2 g per hour. This total excretion is actually larger than the amount broken down by maximal oxidation: 7 g each hour (calculated above). The rate (b) increases with increasing temperature, with increased metabolism (thyroid hormones, dinitrophenol), and decreases under the influence of enzyme inhibitors. Hepatic alcohol dehydrogenase metabolises alcohol to acetaldehyde, which is oxidised to acetate by aldehyde dehydrogenase in the mitochondria. Acetate is then oxidised to carbon dioxide and water, primarily in the peripheral tissues. Fructose increases b. Both enzymes are dependent on nicotinamide adenine dinucleotide (NAD+). One mole of alcohol oxidised to acetate produces 2 moles of reduced nicotinamide adenine dinucleotide (NADH). The sum [NAD+ + NADH] is constant. Hepatic alcohol oxidation causes [NADH] to rise, so that NADH inhibition becomes the rate-limiting factor for oxidation. There are two other enzymes, apart from hepatic alcohol dehydrogenase, that can oxidise alcohol. These are catalase and MEOS (Microsomal Ethanol Oxidation System). A small amount of alcohol dehydrogenase is found in the gastric mucosa. 4. The respiratory quotient and R RQ is the hypothetical, metabolic ratio between carbon dioxide output and oxygen consumption of all the cells of the body. RQ is an indicator of the type of foodstuff metabolised. R is the ventilatory ratio between CO2 output and O2 uptake for the person quantified by gas exchange equipment. Respiratory steady state is a condition where R equals RQ, and the gas stores of the body are unchanged. See Chapter 16. Compared to the oxidation of carbohydrates (RQ = 1), fat oxidation has a distinctly low RQ (0.7), and protein is oxidised with a RQ of 0.8. Carbohydrates are rich in oxygen compared to the minimum in fats. Overfeeding with carbohydrates results in a partial conversion to fat. The corresponding release of oxygen from carbohydrates diminishes the oxygen uptake, and R becomes larger than one. The diminished glucose metabolism during fasting and in diabetics lowers the R towards 0.7, because of the increased conversion rate of fat. Hyperventilation decreases the amount of exchangeable CO2 in the large body stores, without altering oxygen uptake. The tissues and blood cannot store additional oxygen. As a consequence the V°CO2 / V°A -ratio (= FACO2) is reduced. This implies a fall in PACO2 and in PaCO2. R is distinctly increased during hyperventilation - often up to 2-3. Hypoventilation reduces R towards zero at apnoea. Metabolic acidosis is characterized by low pH and by negative base excess in the extracellular fluid (Chapter 17). A high pH and positive base excess characterise metabolic alkalosis. Metabolic acidosis is compensated by hyperventilation implying a rise in R, and metabolic alkalosis is compensated by hypoventilation with a fall in R. R does not change when a person on a mixed diet (RQ = 0.83), or when a person on a high fat diet (RQ = 0.7) exercises moderately, because the fat combustion dominates. R will fall, however, when a person on carbohydrate rich diet (RQ = 0.96-1) works for hours. Strenuously heavy exercise implies a substantial, initial rise in R (R>3), because the lactate liberated will release CO2, which is then eliminated in the lungs in much larger volumes than oxygen is taken up. Glycogen: (C6H1005)n + 6n 02 = 6n CO2 + 5n H20 , that is RQ = 1. Glucose: C6H1206 + 6 02 = 6 CO2 + 6 H20, that is RQ = 1. The enthalpy released per mol of glucose is 2826 kJ. One mol of glucose has a mass of 180 g, and 6 mols of oxygen have a volume of (6 × 22.4) = 134.3 l STPD. The enthalpy per g of glucose is thus 2826/180 = 15.7 kJ/g, and the energy equivalent, which expresses the energy with respect to the oxygen consumed, is 2826/134.3 = 21 kJ per l STPD. The dietary protein-nitrogen is equal to the nitrogen excretion in the urine when the person is in nitrogen balance. Protein-retention during growth, training, protein-rich diet, pregnancy and reconvalescens are called positive nitrogen balance (not urea accumulation in uraemia). Protein-loss during inactivity, bed rest, fever, blood loss, burns and lesions is called negative nitrogen balance. The net mechanical efficiency (Enet) is the ratio of external work rate (N × m/s = J/s) to net chemical energy expenditure (J/s or Watts) during work. Enet is 20-25% in isolated muscles and also in humans during aerobic cycling. Its size increases with the amount of training, because the untrained individual does not use the muscles effectively. Legwork has the largest Enet, since arm work necessitates fixation of the shoulder belt. The work rate is measurable with a cycle-ergometer (Eq. 20-5). The predominant source of energy is oxidation of fuel in the mitochondrion. Hereby, high-energy compounds such as creatine phosphate and ATP are formed. Glucose is oxidised by nicotinamide-adenine-dinucleotide (NAD+), so by glycolysis two pyruvate molecules are formed in the cytosol, transported to the mitochondrion, and transformed to a Co-enzyme-A derivative (acetyl-CoA), which then is involved in theTri-Carboxylic Acid (TCA) cycle (Fig. 20-2). Provided a certain oxygen flux from the lungs to the mitochondria is present, the electron transport chain (the glycero-phosphate shuttle) will reoxidize (NADH+H+) and FADH2 to NAD+ and FAD (Fig. 20-2). In the glycolysis, one glucose molecule is converted to 2 molecules of pyruvate, with the other products being 2 ATP and (2 NADH + H+). Through the oxidation of pyruvate in the TCA-cycle, three (NADH+H+), one FADH2, and one GTP are formed. If complete oxidation occurs in the glycerophosphate shuttle of the mitochondrion, one NADH equals 3 ATP, and one FADH2 equals 2 ATP. Since the NAD+ reduced in the glycolysis is cytosolic, it usually equals 2 ATP only, depending on the shuttle used. When pyruvate is transformed to acetyl-CoA, one molecule of (NADH++H+) is formed. The total production by use of the glycero-phosphate shuttle in oxidative phosphorylation is 36 ATP molecules per glucose molecule (6 from the glycolysis, 6 from the transformation and 24 from the TCA cycle). - If the malate-aspartate shuttle is used, a total of 38 ATP molecules are formed per molecule of glucose oxidised. Oxidation of one glucose molecule typically implies the use of six oxygen molecules. Accordingly, the P: O2 ratio is 36/6 = 6, which is equal to a P:O ratio of 36/12 = 3. The free fatty acids (FFA) from the cytosol (intramuscular or extramuscular origin) are transformed to acetyl-CoA (Fig. 20-2). The pyruvate production rises with increasing glycolysis rate, and pyruvate is the substrate for alanine production. Alanine is liberated to the blood and its concentration increases linearly with [pyruvate] during rest and exercise. During anaerobic conditions - an insufficient oxygen supply - (NADH + H+) is reoxidized by the pyruvate- lactate reaction, and the glycolysis continues. The anaerobic ATP production does not block the aerobic ATP production, but functions as an emergency supply. Fig. 20-2: Biochemical pathways for ATP production. The largest rise in blood [lactate] takes place at work intensities above 50% of the maximum oxygen capacity. Lactic acid is a fixed acid - in contrast to the volatile H2CO3 - produced during exercise, and in a muscle cell with a pH of 7 such an acid is essentially totally dissociated (pK = 3.9). Since the proton associated with lactate production reacts immediately with bicarbonate within the cell, its CO2 production must increase by one mol CO2 for each mol of bicarbonate buffering lactic acid. Lactate accumulates in the muscles and blood, if the glycolysis proceeds at a rate faster than pyruvate can be utilised by the mitochondria, or if (NADH + H+) is not reoxidized rapidly enough. We posses 100 mmol of glucose (stored as glycogen) per kg of wet muscle weight, or 3.5 mol in the muscle tissue. Muscle tissue does not contain glucose-6-phosphatase. Our normal 5-l of circulating blood only contains 5 mM, or as a total 25 mmol (5 g) of glucose. During exercise the muscle uptake of glucose increase considerably, but the blood [glucose] does not fall. The blood [glucose] is kept normal by an increased flux of glucose from the liver (Fig. 20-3). Fig. 20-3: A schematic overview of carbohydrate metabolism. 1. With increasing intensity and duration of exercise, the sympatho-adrenergic activity and the blood [catecholamines] increase. This is a strong stimulus to the hepatic glucose production. The liver contains 50-100 g of dynamic glycogen. This liver glycogen is easily broken down into glucose by glycogenolysis and released to the blood. Any fall in blood [glucose] during exercise will increase the blood [glucagon] and decrease [insulin] toward zero. Glucagon is bound to hepatocyte receptors, and via cAMP a glycogenolytic cascade is started (Fig. 20-3). Hereby the hepatocytes produce large amounts of glucose, sparing muscle glycogen and delaying the onset of fatigue. The lack of insulin inhibits the glucose transport across the cell membranes. 2. Glucose is also produced by gluconeogenesis in the liver from glycerol, lactate, pyruvate, and glucogenic amino acids. The gluconeogenesis is stimulated by pituitary ACTH and by cortisol from the adrenal cortex. With prolonged exercise the blood [glucose] will fall at the end, when hepatic and muscle glycogen stores are depleted, and the compensating gluconeogenesis is also running out of energy sources. 3. Complete exhaustion is delayed considerably in trained athletes, because they utilise lipids, so the glycogen stores are spared by oxidation of free fatty acids (FFA). Skeletal muscles contain lipid stores (20-g triglycerides/kg wet weight or 700 g in a person with 35-kg muscles). A standard 70-kg man also contains extramuscular fat stores of triglycerides (15 kg). Sympathetic activity and catecholamines increase lipolysis (i.e., hydrolysis of the stored adipose tissue to FFA and glycerol) via activation of adenylcyclase, increase in cAMP, phosphorylation and activation of the hormone sensitive lipase. Increased blood [lactate] and glucose intake reduces lipolysis during exercise. The fat stores are the ideal energy stores of the body, because a large quantity of ATP is available per g; this is due to the relatively low oxygen content of lipids - the point being that the necessary oxygen is inhaled at request. At rest we have a slow turnover of muscle protein, but during exercise alanine is released in appreciable amounts by transamination of pyruvate in the muscle cells, and the blood [alanine] is doubled - without any important change in other amino acids. Alanine is produced via the pyruvate-alanine cycle, and the amino groups are from valine, leucine and isoleucine. The blood to the liver transports the muscle alanine, where its carbon skeleton is used in the gluconeogenesis. The blood [alanine] also stimulates the pancreatic islet-cells to increased glucagon secretion. Glucagon activates the glycogenolytic cascade (see above) in the liver cells, further stimulating glucose output from the liver. These are the two factors in the alanine-liver cycle of exercise. The ventilatory, the cardiovascular and the metabolic systems are coupled, and determined by the following factors: The primary factor is the size of PaO2, but the blood oxygen store is of similar importance in keeping PaO2 as high as possible. The blood oxygen store depends upon the haemoglobin concentration, the haemoglobin-oxygen affinity incl. 2,3-DPG, temperature, and PaCO2. The total oxygen flux to a certain population of mitochondria also depends upon the bloodflow (ie, cardiac output, muscle bloodflow, lung perfusion etc.). Indirect measures of enthalpy (MR in kJ/min) are easily applicable both at rest and in an exercise setting. Expired air is collected in a Douglas bag (volumetric principle) for subsequent air analysis, and the volume of oxygen consumed per min is calculated. It is convenient also to determine the carbon dioxide production in the same period, because their ratio is the respiratory quotient (RQ). A person on a mixed diet has a RQ of 0.83 and a heat energy yield of 20 kJ per l or 0.45 kJ/mmol of O2. The metabolic rate (in kJ/min) is calculated by multiplying the estimated volume (l/min) of O2 consumed with 20 kJ per l. The heat energy yield varies with RQ and is found in a table (see Symbols). A metabolic ratemeter - a spirometer (Fig. 13-1) with CO2 absorber - is practical for determination of oxygen uptake. A more detailed calculation of the metabolic rate is performed as shown with Eq. 20-6 and 20-7. Disadvantages of indirect calorimetry are that it ignores the O2 debt, and that the method depends upon maintained nitrogen balance and gas stores. The total output of heat energy from the body is most precisely measured in a whole-body calorimeter. The Atwater-Rosa-Benedict's human calorimeter has been used to verify the first law of thermodynamics in humans. The heat energy delivered from the chamber is only equal to the metabolic rate (MR), provided the external work is zero, and neither equipment nor the human body alters temperature. Fig. 20-4: The human calorimeter combined with a metabolic ratemeter. The major single factor is muscular activity, which can increase MR with a factor of 20 even for hours in marathon running. Inactive persons can have a daily MR of 9600 kJ, whereas heavy occupational labour requires 20 000 kJ (20 MJ). Dietary intake can increase MR by 20-30% (see Specific Dynamic Activity, below). Increased energy demand in heart and lung diseases, rapid growing cancer will increase MR importantly. Energy is also lost in other disease states such as proteinuria, glucosuria, ketonuria, diarrhoea, and exudate loss (of plasma) through lesions in the skin or in the mucosa. An extra physiologic energy loss takes place during pregnancy and during nursing. Deposition of heat energy in the body (as in fever and hyperthermia) can increase MR. No work is done under basal conditions, so that all energy is ultimately liberated in the body as heat energy. The liver and the resting skeletal muscles account for half of the basal metabolic rate. Measurement of the basal metabolic rate (BMR) requires the subject to be awake in the morning, fasting and resting horizontally. The ambient temperature must be neutral, which is the temperature at which compensatory activities are minimal. Prediction tables for BMR in different races are available, and the variables are age, sex, height, and weight and thyroid function. BMR is rarely used for diagnosis of thyroid disease, because radioimmunoassays (Chapter 26) for thyroid hormone analysis are specific and uncomplicated in use. The surface law states that the BMR per body surface area is much more uniform than the BMR per kg of body weight in individuals of the same species but of different form and size. The best expression is the BMR per kg of lean body mass. The lean body mass is the fat free mass. Among different animal species the large animals (elephants) have the smallest relative surface area (ie, surface area per kg), so elephants must have small BMR per surface area compared to mice. This is because the surface-volume ratio decreases with increasing body weight. Besides, small animals also have a thin body shell. The body surface area is estimated with Eq. 20-8. BMR decreases with age in both sexes (Fig. 20-5). Fig. 20-5: The basal metabolic rate in females and males decreasing with age. The female surface-related BMR values are approximately 10% below the male values throughout life. Let us compare a female and a male both 21 years of age. The female has a Height of 1.68 m, weight 58 kg and a surface area of 1.66 m2, whereas the male values are:1.8 m, 76 kg and 1.95 m2. Calculations from the values read at Fig. 20-5 result in BMRs of 70 and 90 Watts, respectively. Now, let the couple live for 50 healthy years maintaining height and weight. At the age of 71, their BMRs are reduced to 60 and 75 Watts, respectively. Intake of meals as such increases metabolic rate. This is the specific dynamic activity of the diet (SDA) or dietary thermogenesis. SDA is less than 10% of the intake energy for carbohydrates and for fat, but 30% for proteins (Fig. 20-6). Fig. 20-6: Dietary thermogenesis or so-called specific dynamic activity of foods. Glucose loaded person forms glycogen and fatty acids out of glucose within an hour, even before the glucose can be oxidised. Accordingly, the SDA caused by glucose can be due to an obligate formation of glycogen and fatty acids. The thermogenic response to carbohydrate seems to include a muscular component activated by adrenaline via b2-receptors and a non-myogenic component activated by noradrenaline (NA) via b1-receptors. Proteins have no SDA in hepatectomized animals, so hepatic intermediary processes must cause the SDA of proteins. These intermediary processes include formation of urea from NH4+, breakdown of amino acids etc. In general, SDA can also be related to mass action due to increases supply of nutrients, and to temperature increase by the activity (increases the rate of all enzymatic processes). Native diets in Africa and the Orient are rich in fibre, which are plant substances (ie cellulose, hemicellulose A & B, and lignins) resistant to digestion. Dietary fibre has been used in an attempt to cure obesity. Constipation with or without diverticulosis/ diverticulitis of the colon also responds to dietary fibre. The most widespread dietary fibre is cellulose, which is a major component of plant cell walls. Cellulose is a linear glucose polymer, but human intestinal enzymes cannot hydrolyse its b-1,4-linkages. Hemicellulose A is a heteropolymer with linkages between glucose, galactose, mannose, xylose and arabinose (ie, gums or mucilages). Mucilages delay gastric emptying and decrease the rate of intestinal absorption. Hemicellulose B or pectin binds water in the gastrointestinal tract, but in addition salts minerals and heavy metals. Hemicellulose A and B seem to lower LDL concentrations, while maintaining HDL concentrations. Lignins in natural fibres are cross-linked polymers of oxygenated phenylpropane entities. Lignin provides bulk for the faeces because they are difficult to degrade. Dietary fibres reduce postprandial blood glucose and insulin concentration. Delay in gastric emptying caused by some dietary fibres reduces symptoms of the dumping syndrome. This is an unwanted consequence of large gastrectomies. Following removal of the major part of the stomach, the food pass quickly down the small intestine and elicit distension by nutrients and osmosis, causing a massive sympathetic activity with discomfort. Dietary fibres seem to prevent hiatus hernia by softening the food bolus and decrease of the swallowing effort. Softening of the faecal bulk with decreased defaecation strain seems to reduce the frequency of haemorrhoids. Overconsumption of dietary fibre can produce adverse effects with increased flatulence, diarrhoea and intestinal discomfort. Fig. 20-7: Continuous fasting leads to numerous serious complications or death. Fasting is a total stop of food intake. After 12 hours of fasting, conditions are optimal to measure BMR or to analyse the chemical composition of blood (fasting blood values are predictable and easy to interpret). The 12 hours are the methodological criterion for the correct minimum BMR, but continued fasting for day’s results in a much lower value (65% of BMR). Following the first 2 weeks of hunger is the normal body weight reduced to 85%, whereas the resting MR is stable at 65% of BMR, which is constant to the end of the fasting period (either voluntary or by death). Glycogen stores are broken down in a few days, since only small stores prevail in the liver and muscles. Then urine nitrogen increases as a sign of renewed protein combustion (gluconeogenesis). In general the fat combustion dominates, until the fat stores are used. Healthy people contain 5-15 kg of fat, but monstrous amounts have been recorded in a 540-kg male from Guinness Book of Records. Oxidation of fat stores - including the partial hepatic oxidation to ketonic bodies - implies development of ketoacidosis and a diabetic glucose tolerance test (Chapter 27). Such a hunger diabetes with ketonaemia and ketonuria as in diabetes, have been found in healthy individuals even after only 24 hours of fasting or after extremely fatty meals. Serious illnesses develop after a few weeks of fasting, because the cell structure proteins are broken down (Fig. 20-7). The proteins of the cell nuclei produce uric acid, which accumulate in the heart (cardiac disease) and in the articulations (uric acid arthritis or podagra). Energy balance is a condition, where the energy input equals energy output, so the energy stores of the body are unchanged. A person with a body weight of 70 kg contains 550 MJ of combustible energy (entalphy), and if allowed to eat naturally, at least 10 MJ is consumed every day. If the person is fasting for some days he will lose body weight and his metabolic rate will fall to 6.6 MJ daily, so a certain input control is hereby documented. The loss in body weight is rapidly compensated when feeding is resumed. If enough food is available the person automatically eat more and more (towards a doubling) with increasing workload (MJ/day), so also a certain output control is documented. The internal feedback signals operating in this output and input control are uncertain. Signals from gastrointestinal centres inhibit the feeding or hunger centre in the lateral hypothalamic area through afferent nerves (Fig. 20-8). Chyme in the duodenum containing HCl and fatty acids liberate enterogastrones to the blood (ie, intestinal hormones that inhibit gastric activity and emptying). The enterogastrone family consists of secretin, somatostatin, cholecystokinin (CCK) and gastric inhibitory peptide (GIP). Enterogastrones reduce gastric activity, stimulate the satiety centre (Fig. 20-8), and increase the production of bicarbonate-rich bile and pancreatic juice. A glucose-rich chyme in the duodenum liberates members of the incretin family to the blood. The incretin family consists of gut glucagon, glucagon-like peptide 1 & 2, and GIP. incretin produce a rapid rise in insulin secretion, which causes the energy stores to increase. The hunger and satiety centres operate reciprocally. The lipostatic theory explains the constant body weight by liberation of a lipostatic, satiety peptide called leptin (ie, thin) from fat tissue. The plasma concentration of leptin is recorded by hypothalamic satiety centres, and seems to reflect the size of the body fat stores or the body fat percentage. Obese patients, often with excessive high plasma leptin concentrations, reduce their leptin concentrations by Banting. Some patients may lack the normal sensitivity to leptin. The leptin molecule is large (16 kDa) and it probably must pass the large fenestrae of the circumventricular organs in order to reach the hypothalamic control centres (Fig. 20-8). The plasma leptin concentration is highest at night. Also thermoregulatory signals from cold and heat receptors and the plasma concentrations of nutrients may stimulate the satiety centre. In workers, a minimal work activity threshold must be passed in order to trigger the hypothalamic weight control, but above this threshold eating increases proportional with the workload, and the body weight is constant. If the work rate is extremely high as in marathon training, the hypothalamic control is broken, and the dietary intake and the body weight cannot cope with the high combustion. The body weight falls drastically, which is a certain sign of overtraining. The cybernetics of appetite control is not only feedback factors. As in all human behaviours, cerebral feedforward factors can dominate. Cerebral feedforward factors are exercise habits, eating habits, social inheritage, and they can be of extreme importance to the individual. Fig. 20-8: A schematic overview of the regulation of food intake. The hypothalamic-feeding centre is located in the lateral region, whereas the satiety centre is medially located in the hypothalamus. The hypothalamus controls food intake and metabolism, mainly by autonomic effects on the islets of Langerhans (secreting insulin, glucagon, pancreatic polypeptide, and gastrin), hepatocytes and adipocytes. Neuroendocrine-behavioural disturbances seem to be involved in abnormal eating patterns such as anorexia nervosa, bulimia nervosa and obesity. We seem to regulate our appetite by a combination of negative feedback and essential feedforward factors. Vitamins are essential organic catalysts in the diet, necessary for normal metabolic functions in humans, but not synthesized in the human body. Essential catalysts refer to the fact that lack of the compound in the diet results in a clearly demonstrable disorder in humans. Vitamins A, D, and K are lipid soluble, so they follow the lipid absorption to the liver, where they are stored. Accordingly, any type of lipid malabsorption results in vitamin deficiency of these vitamins. The vitamin B complex (B1, B2, B6, B7, B12, folate) and vitamin C are water-soluble. Since they are only stored in minimal amounts, vitamin deficiency develops rapidly. Exceptional is the enormous vitamin B12 store in the human liver, so pernicious anaemia takes years to develop. This paragraph deals with 1. Starvation with marasmus, 2. Vitamin deficiencies, 3. Alcohol intoxication, 4. Obesity, and 5. Hyperuricaemia and gout. Lack of all elements in the diet of a child - or universal starvation - leads to marasmus. Marasmus is often complicated by deficiencies in vitamins and essential minerals. Marasmus is common throughout the third world, because when breast-feeding stops, the child must try to survive on an insufficient diet. The body weight decreases, the fat stores disappear, muscle wasting leads to thin limbs that do not grow in length, and infants and children look like aged persons (Fig. 20-9). Fig. 20-9: The child has lived through periods of universal starvation alternating with periods on a diet mainly consisting of cassava. The result is a combination of marasmus and kwashiorkor. The abdomen is tremendously distended, because of hepatomegaly, flaccid abdominal muscles and possibly oedemal fluid in the abdominal cavity (ascites). The short half-life of the intestinal mucosal cells makes them especially sensitive to lack of nutrients, so villous atrophy develops with malabsorption and diarrhoea. Marasmus is unexpected in the rich part of the world. However, this is not so. Malignant tumours and severe cardiopulmonary disease imply an enormous loss of energy, and terminal weight loss is unavoidable even where the diet is supposed to be sufficient (hospitals and other institutions). The basal metabolic rate is low and the core temperature is also controlled on a lower than normal level. The heart rate and arterial blood pressure is also low. Haemopoiesis is deficient and anaemia prevails. The immune defence system is impaired and the patient suffers from numerous infections. Children suffer from growth failure, and brain development is probably affected. Children fed with a diet deficient in protein alone (essential amino acids) develop protein deficiency or kwashiorkor. Following breast-feeding, these children subsist on a protein-poor diet rich in carbohydrates (eg, cassava). Thin limbs, hypoproteinaemia and ascites (Fig. 20-9) characterise kwashiorkor. The large liver is fatty, because there is carbohydrates enough to provide the hepatocytes with lipids, but the lack of protein makes the production of lipid transporting proteins (apoproteins) inadequate. In spite of the effort from all international institutions concerned, the fraction of the global population falling below the minimum food intake defined by WHO, is increasing, and has done so for years. Vitamin A (retinol) and its analogues are termed retinoids. Vitamin A occurs naturally as retinoids or as a precursor, b-carotene, in vegetables. Infants fed with cooked milk in developing countries, and adults suffering from chronic disorders with fat malabsorption may develop vitamin A deficiency. Retinoids have the following three effects: 1. Retinoids are an important constituent of the photosensitive pigment of the retina and enhance night vision. The 11-cis-retinal is the aldehyde of vitamin A1. Retinal combines with the glycoprotein opsin to form rhodopsin (visual purple) in the retina during darkness. Vitamin A deficiency implies a massive fall in the number of rhodopsin molecules in the outer segment of the rods. This impedes dark adaptation and night blindness occurs. 2. Retinoids stimulate cellular growth and differentiation. Retinoids convert keratin-producing cells into mucus-producing cells, transcribe new mRNA and encode for new cell proteins, so a more differentiated cell type develops. 3. Vitamin A promotes growth of the skeleton. Lack of vitamin A causes diminished vision in dim light, followed by night blindness, and eventually blindness. Lack of vitamin A leads to squamous metaplasia of the conjunctiva and glandular epithelium. The tear ducts are occluded by the metaplasia, causing eye dryness (ie, xerophthalmia), and occluded sebaceous glands cause follicular hyperkeratosis. Vitamin A deficiency causes growth retardation. Retinoids are used in cystic acne and in psoriasis. Vitamin A in normal dosage has been proposed as an anticarcinogen. Excess intake may be teratogenic. Thiamine - as the majority of the B complex - is found in green vegetables, milk and liver. Thiamine is the co-factor for many enzymes in the glycolytic pathway. Thus lack of thiamine leads to inadequate glucose metabolism with accumulation of vasodilatating lactate and pyruvate. The peripheral vasodilatation leads to oedema. The increased work of the heart eventually develops into cardiac failure, which increases the venous stasis and worsens the oedematous state. Thiamine deficiency (beri-beri) is found in persons consuming polished rice (classical beri-beri), in chronic alcoholics, and in marasmus (see above). Dry beri-beri is symmetrical polyneuropathy (ie, paresthesia, weakness, heaviness, and paresis of the legs). CNS involvement with ischaemic damage results in the Wernicke-Korsakoff syndrome (ie, ataxia, confusion and ophtalmoplegia). Wet beri-beri describes thiamine deficiency with oedemas of the legs, pleural effusions and ascites. - These disorders respond immediately to thiamine treatment. Vitamin B2 (riboflavin) deficiency Riboflavin is widely distributed in animal and vegetable foods. Riboflavin is destroyed by ultraviolet light but thermostable and not destroyed by cooking. In the human body riboflavin is converted into flavin mono- and di-nucleotides. These compounds are of crucial importance in the electron transport chain. Riboflavin deficiency is frequently only part of a combined vitamin B deficiency, but the classical manifestations are lesions around the natural openings: 1. interstitial keratitis of the cornea with vascularisation, 2. seborrhoic dermatitis (face, vulva, and scrotum), 3. angular stomatitis (ie, cheilosis or fissures at the angles of the mouth), and 4. glossitis. These lesions respond to riboflavin usually given parenterally as a vitamin B complex. Vitamin B6 deficiency Vitamin B6 activity is found in three compounds found in both vegetable and animal foods: pyrodoxine, pyridoxal, and pyridoxamine. Pyridoxal phosphate is a co-enzyme for transaminases, carboxylases (formation of the neurotransmitter GABA) and other enzymes. Drugs like the antituberculosis drug, isoniazid, and the copper-chelating agent, penicillamine, are B6-antagonists. Certain types of polyneuropathy including the CNS and anaemia with saturated iron-stores respond to vitamin B6 therapy. Vitamin B7 deficiency Niacin deficiency or pellagra (ie, roughs skin) is recognized by the combination of the 3 diagnostic Ds: Dermatitis, diarrhoea, and dementia. Light exposed areas exhibit dermatitis with rough scales. The diarrhoea is colonic and watery. Cortical atrophia and degeneration of myelinated tracts in the spinal cord cause the dementia. Niacin is involved in the formation of nicotinamide adenine dinucleotide (NAD) and its phosphate (NADP). These molecules are important in many oxidation/reduction reactions of the intermediary metabolism. We consume niacin found in different types of grains (poor content in maize), and our endogenous synthesis is from tryptophan found in meat, eggs and milk. Pellagra is seen in malnourished alcoholics, food faddists, and in patients with the carcinoid syndrome, where most of the tryptophan is used for serotonin synthesis. Vitamin B12 deficiency Vitamin B12 is almost ubiquitary in animal foods (meat, fish, eggs and milk) but not in vegetables, so dietary deficiency is only found in extremely rare cases of vegetarianism, starvation or anorexia nervosa. Malabsorption disorders (ie, pancreatitis, coeliac disease) seldomly result in biologically consequential B12 deficiency. Vitamin B12 deficiency causes pernicious anaemia (Chapter 8). Below is described the absorption of the vitamin in the terminal ileum (Fig. 20-10). Fig. 20-10: The mechanism of normal vitamin B12 (cobalamin) absorption in the terminal ileum and storage in the liver. The intrinsic factor-cobalamin complex is resistant to pancreatic proteases, and is normally carried along the gastrointestinal tract to specific receptor proteins on the mucosal surface of the terminal ileum. The complex is recognized and bound to the receptor. The free vitamin B12 enters the enterocyte, and the intrinsic factor remains in the lumen. Vitamin B12 exits from the enterocyte by facilitated or active transport, and appears in the portal blood bound to the glycoprotein, transcobalamin II (Fig. 20-10). The hepatocytes clear the portal blood for vitamin B12 by receptor-mediated endocytosis. The hepatic vitamin B12 store is enormous in healthy individuals. An average value of 5 mg stored vitamin B12 must be compared to a daily requirement of 1 mg. Transcobalamin II is the main carrier in delivering vitamin B12 to the red bone marrow, although most of the vitamin B12 is bound to transcobalamin I and III. Folic acid deficiency Foliates are present in leafy green vegetables such as spinach and broccoli, and in organs such as kidney and liver. Excessive cooking destroys much of the food folate. Pregnancy increases the requirement for folate up to tenfold. Folate is absorbed in the small intestine, and transported to the cells via the blood plasma. Folate deficiency with poor diet for a few months’ results in megaloblastic anaemia and glossitis because the stores of folate are small compared to the enormous liver storage of vitamin B12 (Chapter 8). Vitamin C deficiency (scurvy) Vitamin C or ascorbic acid is a reducing substance found in fresh fruit and vegetables. Humans cannot synthesise ascorbic acid from glucose as several animals. Ascorbic acid contributes in controlling the redox potential of the cells. Ascorbic acid is necessary the hydroxylation of proline to hydroxyproline. This is the single process necessary for the production of collagen in all tissues including the vessel walls. Vitamin C deficiency (scurvy or scorbutus) is found among food faddists and in developing countries, where infants are fed with excessively boiled milk. The patient with scurvy can only produce abnormal collagen without sufficient tensile strength. The capillaries become fragile and bleedings are frequent. They are recognized as bruises of the skin, as haemarthron, as subperiosteal bleedings and eventually bleeding anaemia develops (Chapter 8). Infections are prolonged and the healing of wounds is poor. Infections of the gingiva (gingivitis) leads to loose teeth, and the lack of normal collagen in growing bones results in arrested bone growth. Bottle fed infants must receive daily fruit juice, and for poorly fed adults fresh fruits and vegetables are the best preventive means of avoiding scurvy. There is no advantage in the daily intake of large doses of vitamin C to prevent or improve common cold or cancer. In one controlled clinical trial there was an accumulation of cases with kidney stones. Rebound scurvy may occur following a sudden stop of the intake of large doses of vitamin C. Vitamin D deficiency is described in Chapter 30. Hypervitaminosis D is caused by excess consumption of vitamin preparations. This leads to hypercalcaemia, nephrolithiasis, nephrocalcinosis and ectopic calcification of other organs including premature arteriosclerosis. Vitamin K deficiency Vitamin K occurs in two forms in nature. Vitamin K1 is produced in plants, and intestinal bacteria in animals synthesise vitamin K2. Insufficient dietary intake of vitamin K is infrequent, and occurs occasionally in the chronically ill patient such as cases of anorexia nervosa. Fat malabsorption is accompanied by vitamin K deficiency, because vitamin K is fat-soluble. Newborn babies sometimes suffer from vitamin K deficiency, because the molecule only crosses the placental barrier with difficulty, and because the sterile gut of the baby cannot produce vitamin K2. Destruction of the intestinal bacteria by long term antibiotic treatment may also lead to vitamin K deficiency. Vitamin K deficiency can lead to terminal bleeding. This is because vitamin K normally activates four clotting factors: prothrombin, factor VII, factor IX, and factor X. These four proteins probably receive Ca2+ binding properties from vitamin K (see Chapter 8). Therapy with intramuscularly administered vitamin K is rapid and effective. Vitamin E deficiency Vitamin E (a-tochoferol) is found in fish, fish oil and vegetable oil from Soya beans and corn. Vitamin E is an antioxidant. Vitamin E protects the phospholipids of the plasma membrane against peroxidation by free radicals produced by the cell metabolism. Prolonged vitamin E deficiency is rare, but leads to CNS lesions, haemolytic anaemia, and muscle disorders. Patients with fat malabsorption or patients receiving parenteral nutrition may develop vitamin E deficiency. The sequence of events in acute alcohol intoxication proceeds with an increasing sense of warmth, flushing of the face, dilated pupils, dizziness and euphoria. There is a general sense of well being with unjustified optimism and the feeling of increased strength and energy. The subject shows a boisterous behaviour with increased psychomotor activity, which is clumsy, and social inhibitions are dissolved. Negative consequences of alcohol abuse are arrests, automobile accidents, and deleterious effects upon job performance and chronic health problems. Alcohol interferes with the arrangement of molecules (ion channels, receptors, the GABA-benzodiazepine-channel etc.) in the lipid bilayers of the cell membrane. With increasing intoxication the symptoms and signs of CNS depression become apparent. The subject becomes drowsy, argumentative, angry or weepy, and eventually he is vomiting and complaining of diplopia. Later an examination reveals areflexia, loss of muscular tension, loss of sphincter control, rapid heart rate and respiratory frequency, decreasing arterial pressure and mean arterial pressure leading to shock. The subject develops hypothermia and increasing stupor, anaesthesia, coma or death. The intoxication depresses the myocardium and dilates the peripheral vessels. This is why the MAP is falling together with cardiac performance. Some alcoholics benefit from treatment with disulfiram (Antabuse) or similar drugs. Antabuse inhibits aldehyde dehydrogenase, which results in poisoning from accumulated acetaldehyde. Overeating is related to social patterns and constitutional family traits. Obesity or adiposity implies the excess storage of fat, and is defined by WHO as an actual body weight exceeding the ideal weight by more than 20% (if not explained by an above-average muscle and bone mass). The diagnosis is frequently set by inspection of the undressed patient (Fig. 20-11). The fatty stores of the patient in Fig. 20-11 are clinically acceptable. The ideal weight is the weight associated with the highest statistical life expectancy. The Broca index is a popular and easy method of determining the recommended weight. Brocas index is the predicted body weight in kg, which equals the height of the person in cm minus 100 for males and 110 for females.Fig. 20-11: The ideal weight and clinically acceptable fatty stores. Obesity is also established in another way by the help of the body mass index (BMI). BMI is the weight of the person in kg divided by the height (in m) squared. The normal range is 19-25 kg per square metre (Fig. 20-11). Marginal overweight is defined as a BMI between 25 and 30 kg* m-2. Obesity is defined as BMI above 30 kg* m-2, which corresponds to body weights 20% above ideal weight. Obesity results from a long-term excess of nutritional intake relative to the energy liberation. There are at least three types of obesity: genetic, overeating and inactivity induced.1. Genetic obesity. Genes account for quite some cases of obesity. Genes seem to be causative in 2/3 of all cases of obesity in a lifetime. Genetic movement oeconomists may explain many cases of obesity including familiar obesity, but weight gain does not occur in all pairs of mono- or di-zygotic twins. - Hyperplastic fatness (too many adipose cells) is often found in babies from the rich part of the world, whereas many adults have hypertrophic obesity, which is caused by too large adipose cells. 2. Overeating. Intake of poor food, dominated by sweet-fat combinations, explains other cases of obesity. Sugar and fat eaters need not eat very much in order to develop obesity, if they live with a marginal motility pattern. In any type of obesity the low physical activity or inactive life style is typical. 3. Inactivity. The major factor in obesity is physical inactivity. Persons who exercise can increase their metabolic rate by a factor of 10-20 several hours a day. The second choice of obese persons is to reduce the dietary intake of nutrients. A reduction to half the usual amount of food would be a short, heroic and probably futile project, as well as inefficient, when compared to a metabolic factor of 10-20 during exercise. Inactivity, defined as a low fitness number (ie, a maximal oxygen uptake below 34 ml * min-1*kg-1), is probably involved in western life-style obesity. Obesity is the fate of people dominated by their parasympathetic activities and minimising the use of the sympathetic nervous system. The mortality of a male population increases dramatically with falling fitness (Fig. 18-13). Obese people have a small dietary thermogenesis, because they avoid physical activity with a high metabolic rate in skeletal muscle and in adipocytes. Decreased sensitivity to leptin has been described in obese patients. Rare cases of obesity are caused by hormonal, metabolic diseases (insulinoms, hypercorticism, diseases of the thyroid gland, hypothalamic lesions etc). For persons with insulinoms the high food intake (hyperphagia) can be a question of life and death. Obesity and interrelated risk factors Obesity is clearly a risk factor. A risk factor is an epidemiological term for conditions statistically correlated with shortened life expectancy. Obesity paves the way for maturity-onset (type II) diabetes, atherosclerosis, hypertension, myocardial infarction, and stroke. Type II diabetes is linked to obesity, perhaps because the increased weight or the rise in blood glucose concentration stimulates insulin secretion. A period with high concentration of insulin in the blood plasma decreases the number of insulin receptors on the membranes of muscle and adipose cells (insulin resistance or glucose intolerance). More insulin must be produced from the beta cells of the pancreatic islets, and finally the b-cells are exhausted and a diabetic condition without insulin production developed. Body weight reduction ameliorates the glucose intolerance. Atherosclerosis, acute myocardial infarction, hypertension, hypercholesterol-aemia, gall-stones, low concentrations of HDL, hyperuricaemia, gout, osteoarthritis of the hip and knee joint, and restrictive lung disease are all related to obesity (Fig. 20-12). Fig. 20-12: Adipose patient with numerous complications. There is also an increased incidence of depression, psychological and social problems, intertriginous dermatitis, hernias, impotence, and thrombophlebitis in obese patients. Amenorrhoea and oligomenorrhoea, reduced fertility is common among premenopausal obese females. Therapy of established obesity is a frustraneous and highly resistant task. The current therapy of adiposity, including drugs, diet and behavorial modification with exercise, is ineffective - often due to the lack of motivation. 1. Anti-obesity drugs are either centrally or peripherally active. The centrally active drugs either act on catecholamine neurotransmitters (amphetamines), or they act on serotoninergic neurons in the CNS. Initially, all these drugs reduce food intake, and some of them also increase the metabolic rate. Amphetamines, “holiday pills”, were the first centrally active anti-obesity drugs developed, but there abuse potential is a definite contraindication. The peripherally active anti-obesity drugs, such as acarbiose, have only modest effect in controlled clinical trials. Acarbiose is an amylase inhibitor, which reduce the digestion of sucrose. The lipase inhibitor, tetra-hydro-lipostatin, blocks the intestinal digestion of lipids, but has only a marginal effect on obesity. From a physiological point of view there is little perspective in new development of anti-obese drugs, because any reduction in nutritional input - even a 10% reduction - is futile. 2. Diet. Most popular, weekly journals publish up to three miraculous diets for obese persons in each issue. This is a contradiction with only marginal possibilities of success. Even a 5-10% reduction in dietary input is experienced as self-torture and tolerated only for a short time. There is no alternative to a healthy mixed diet with a sufficient amount of dietary fibre (see above). 3. Behavioural therapy with exercise. The single factor that can cure obesity is a balanced degree of exercise. Exercise can increase metabolic rate from typically 70 Watts at rest to 700 (eg, walking, dancing, sexual intercourse) or 1400 Watts (long distance running). Thus, the most important determinator (factor 10-20) is the increase in metabolic rate from any type of self-induced locomotion. Running is an alternative for younger and middle-aged persons. Older people must walk, if they have the capacity for it. They may prefer walking in a hilly environment in a relaxed way, so the heart is stimulated. Callisthenics, dancing, skiing, swimming, tennis, cycling, golf are alternatives for persons motivated. The important point is to chose the type of exercise, which is enjoyable - in a relaxed way - for the individual concerned. Increased awareness of nutritional and fitness intervention in improving the health status of large population groups is essential. Excessive production or inefficient excretion of uric acid causes hyperuricaemia. Hyperuricaemia is a condition with an abnormally high concentration of uric acid in the blood plasma and ECF (above 0.42 mM). Normal values of serum-urate are 0.2-0.42 mM. The saturation threshold over which urate crystals precipitate is around 0.42 mM for tissues with an acid pH. Hyperuricaemia is asymptomatic for varying periods. When the hyperuricaemia becomes clinically important through recurrent attacks of painful acute arthritis, the condition is called uric arthritis or gout (Fig. 20-13). Fig. 20-13: Gout and its complications are shown to the right. Blockage of the uric acid synthesis by allopurinol is shown to the left. Gout can be primary or secondary. 1. Primary gout. Either increased metabolic urate production, inefficient renal excretion of urate or the two in combination causes genetic or idiopathic gout. Primary metabolic gout relates to two inherited, X-linked enzyme disorders: Hypoxanthine-Guanine-Phospho-Ribosyl-Transferase (HGPRT) deficiency with increased purine synthesis or an abnormally high activity of Phospho-Ribosyl-Pyro-Phosphate -Synthetase (PRPPS). The inherited disorder is exacerbated by diets high in purine or nucleic acids. During purine degradation large quantities of NH4+ are liberated. The acidosis leads to crystallisation of urate. 2. Secondary or acquired gout can also be both metabolic and renal. Intercurrent disease with lysis of cell nuclei and release of nucleic acids increases urate production (eg cancer, psoriasis, and excessive weight loss). Impaired renal excretion leads to secondary renal gout. Most forms of metabolic gout are a result of overproduction of uric acid caused by accelerated purine synthesis from amino acids, formate and CO2, whereas dietary purines play a minor role. Xanthine oxidase oxidises hypoxanthine to xanthine and xanthine to uric acid. Supersaturated body fluids precipitate thin urate crystals in acid environments. The result is an inflammatory reaction, where leucocytes migrate to the crystals and surround them for phagocytosis. The acute attack of gout typically occurs in a male with severe pain in the big toe. The pain attack is also called podagra. The pain responds to therapy and the patient is asymptomatic for a variable period. Following a series of acute attacks of gout, the pain is persistent, because the urate crystals are permanently present in the joints and other tissues. This is called chronic gout. Toes, ankles and knees are frequently affected. Symptoms and signs of gout include hyperuricaemia, tophi and painful arthritis. Symptoms and signs of gout include hyperuricaemia, tophi and painful arthritis, with extremely tender and swollen joints. Complications to gout are increased risk of atherosclerosis, hypertension and renal disease including renal calcification and uric acid stones in the ureter. Persons with hyperuricaemia are at risk, and should be treated with allupurinol (100-300 mg daily) until the plasma-urate is brought down to normal levels (see effect below). Allupurinol is a xanthine oxidase inhibitor. Allupurinol is an analogue to hypoxanthine, but xanthine oxidase (XO) prefers allupurinol as a substrate, so allupurinol is oxidised to oxypurinol (Fig. 20-13). Oxypurinol (alloxanthine) blocks XO, because it binds to the active site on the enzyme. Thus, urate production is inhibited and xanthine/hypoxanthine is accumulated in the blood and ECF. This is fortunate, because these substances are water-soluble and easily excreted in the urine - just as alloxanthine. This is in sharp contrast to the less soluble urate. Uric acid is filtered in the renal glomeruli. Urate is reabsorbed in the proximal tubules by a Na+ -substrate cotransport with a capacity, which is normally far greater than the amount of urate in the glomerular filtrate. Accordingly, the normal urate secretion takes place by active secretion of urate ions in the distal tubules. The organic acid-base secretory system transfer urate ions from the blood to the tubular fluid, but the system has a low capacity for urate. Patients with renal gout suffer from abnormally low distal tubular secretion of uric acid. These patients are treated with uricosuric agents such as probenecid and sulfinpyrazone. These molecules compete for the proximal Na+ -substrate cotransport, so less urate is reabsorbed. Colchicine is a drug that binds to tubulin, a protein in the microtubules of the leucocytes. Hereby, the microtubules disintegrate, which inactivates the leucocytes. Thus, the colchicine prevents the focal infiltration of leucocytes to the damaged tissue and blocks their usual liberation of lactic acid which would further precipitate urate crystals. This effect is slow and not purely beneficial. Weight loss is often indicated during treatment of gout, but a rapid weight loss is risky in hyperuraemic patients, because the cellular destruction liberates nucleic acids and may elicit an acute attack of gout. · The first law of thermodynamics states that the sum of liberated heat energy (-Q) and liberated work (-W') of a system is equal to the fall in internal energy (enthalpy) or heat content (H). The decrease in enthalpy of the human body (-DH) is equal to the fall in potential, chemical energy stored in the body: Eq. 20-1: (-DH) = (-Q) + (-W'). · Entropy is the tendency of atoms, molecules and their energies to spread in a maximum space. The Gibbs energy (G) is the difference between enthalpy (H) and entropy (S) when multiplied with the absolute temperature (T): Eq. 20-2: G = H - T × S. G determines if a certain reaction occurs, since G is minimal at equilibrium. According to the formula, entropy is important at high temperatures, and energy is most important at low temperatures. · The Fick cardiac output equation: Eq. 20-3: Q° = V°O2/( CaO2 - Cv¯O2) · Elimination of alcohol by oxidation. The rate of oxidation is constant (b = 0.0025 permille per min) and is independent of the blood [alcohol]. The absolute amount of alcohol eliminated per minute is thus: Eq. 20-4: Alcohol oxidation (g/min) = (b × r × body weight) The fraction of the body weight which is distribution pool for alcohol is called r (mean-r for females is 0.55 and for males 0.68 kg per kg body weight). · Calculation of work rate on a bicycle ergometer: A measurable blocking force is applied to a wheel with a given radius (r) and with a given rotation-frequency (RPM). The work rate or power (force × velocity) is now determined, because the force is known (N) and the distance per s is (2 × p × r RPM)/60. The work rate is thus measured in J/s or Watts. Work rate = Force * Distance, or Eq. 20-5: Work rate (Watts) = N* (2*p*r *RPM)/60. · Calculation of metabolism by indirect calorimetry: The oxygen uptake and carbon dioxide output is masured volumetrically or gravimetrically together with determination of the nitrogen content in 24 hours urine from the person examined. The urine nitrogen expresses the protein combustion, since protein contains 16% nitrogen. Subtraction of the gas volumes for protein combustion (see data in Symbols) from the total, result in residual volumes of oxygen and carbon dioxide only related to the fat (F g/min) and carbohydrate (C g/min) combustion. Thus F and C can be calculated by solution of two equations with these two unknowns (Eq. 20-6 and -7). By multiplication with the nutritive equivalents for O2 and for CO2 (mmol gas per g in Symbols) the mass balance states: Eq. 20-6: F and C related O2 uptake = (37 mmol/g × C g/min) + (91 mmol/g × F g/min) Eq. 20-7: F and C related CO2 liberation = (37 mmol/g × C g/min) + (64 mmol/g × F g/min). Now the mass of protein, fat and carbohydrate combusted per min is found, and a very precise indirect measure of MR in kJ/min is obtained by multiplication with their energy equivalents (Se Symbols). · Body surface area (BSA) is estimated with the approximation formula of the DuBois family: Eq. 20-8: BSA (cm2) = WEIGHT0.425 (kg) × HEIGHT0.725 (cm) × 71.84 The BMR is normally 45 Watts/m2, so an adult with a body surface area of 1.8 m2 has a BMR of 80 Watts. This corresponds to a daily BMR of (60 × 1440 min × 80 × 10-3) =6912 kJ. I. Each of the following five statements have True/False options: A. Vitamins are essential organic catalysts synthesized in the human body. B. Antibiotics in meats or milk may induce allergy or antibiotic resistance in humans. C. Obesity is the consequence of inactivity alone, and genes are not involved. D. Either increased metabolic urate production, inefficient renal excretion of urate or the combination causes primary or idiopathic gout. E. During therapy with large doses of ascorbic acid, withdrawal may cause symptoms of scurvy. II. Each of the following five statements have True/False options: A. Prolonged use of some anti-obesity drugs may imply serious abuse. B. Osteomalacia does not involve the organic bone matrix. C. Vitamin A deficiency implies a massive fall in the number of rhodopsin molecules in the outer segments of the rods. This impedes dark adaptation and night blindness occurs. D. The epiphyseal plate of the growing skeleton is sufficiently mineralised in rachitis. E. Vitamin K can easily cross the placental barrier. At 7 p.m. a male alcoholic drinks 150 ml of whisky (40 w/v%). His body weight is 58 kg (due to hepatic failure and malnutrition). The fraction of his body weight, which is distribution pool for alcohol, is 0.60. The rate of oxidation of alcohol is reduced to 80% of normal. The rate of oxidation in a healthy person is 0.0025 o/oo per min. At 8 p.m. the patient is involved in a traffic accident and at 9 p.m. his blood [alcohol] is measured to 1.36 g kg-1 (o/oo). The patient states to the police that he has been drinking only the whisky at 7 pm.
Following an earthquake, three adult females are confined under the ruins of their house in an airtight space of 8 m3. The initial pressure is 752 mmHg, the temperature of the water-saturated air is 20 oC (water vapour tension 20 mmHg) and the composition of the atmospheric air is normal. The average oxygen uptake is 190 ml STPD min-1, and the average RQ is 0.83. Assume that PIO2 = 50 mmHg is the survival threshold. 1 Calculate the time period, where they have oxygen enough for survival, provided carbon dioxide could disappear? 2. Calculate the carbon dioxide output per min. 3 Calculate the theoretical amount of CO2, which should accumulate in the time period for survival from 1. Calculate the theoretical CO2 fraction in the airtight space. 4. Explain the consequences of CO2 accumulation. A male, 23 years of age, has a daily metabolic rate of 12 600 kJ (12.6 MJ) and he is eating a mixed diet resulting in a RQ of 0.83. The enthalpy equivalent for oxygen is 0.46 kJ/mmol. His arterial pH is 7.30 and pK for ammonia is 9.3. 1. Calculate the ratio between ammonia and NH4+ in his blood. 2. Calculate his daily carbon dioxide output in mol. 3. Calculate the amount of carbon dioxide eliminated per day in combination with ammonia, assuming one mol/day of ammonia to be involved in the urea production. A 70 kg male, 22 years of age, is in a room, where the temperature is 20o C and PB are 101.3 kPa. The PAO2 is 14.1 kPa, and PAN2 is 78 kPa. The carbon dioxide output is 660 ml STPD per min. The urinary nitrogen excretion is 10 mg per min. The pressure of water vapour in the alveoli is 6.2 kPa, and FIO2 is 0.2093. 1. Calculate the alveolar ventilation and FACO2. Estimate PaCO2, and provide reasoning for a possible hyperventilation in this condition. 2. Calculate the oxygen uptake for this person. 3. Is the pulmonary exchange quotient different from the respiratory quotient (RQ)? Try to solve the problems before looking up the answers. · The first law of thermodynamics states that energy can neither be created nor destroyed but is only transferred from one form to another or from one place to another. · Heat energy is low prize energy. In contrast to ATP energy, it is not available for work in the body. The sum of heat energy generated and work performed is constant and equal to the Gibbs energy. · The oxidation of fuel (carbohydrates, glycerol, fatty acids) to CO2 and water is the primary pathway for generation of energy and subsequent heat energy liberation. Protein can also serve as an important energy source during prolonged exercise. · Alcohol distributes in the total water phase of the body (60% of body weight) within one hour. · Hepatic alcohol dehydrogenase, catalase and MEOS (Microsomal Ethanol Oxidation System) can oxidise alcohol. · The most important elimination of alcohol is by oxidation. The rate of oxidation is constant (b = 0.0025 permille per min) and is independent of the blood alcohol concentration. · The blood alcohol concentration is measured in the unit g per kg (permille) of distribution volume. The absolute amount of alcohol eliminated per minute is thus: (b × r × body weight). · An adult person can oxidise 7 g of alcohol per hour, and at rest only negligible amounts are excreted in sweat, urine and expired air. · During severe exercise by an athlete working in a hot climate, the total excretion of alcohol can be larger than the maximal oxidation (7 g each hour). · The net mechanical efficiency (Enet) is the ratio of external work rate (N × m/s = J/s) to net chemical energy expenditure (J/s or Watts) during work. Enet is 20-25% in isolated muscles and also in humans during aerobic cycling. · The total production by use of the glycero-phosphate shuttle in oxidative phosphorylation is 36 ATP per glucose molecule (6 from the glycolysis, 6 from the transformation and 24 from the TCA cycle). · The total output of heat energy from the body is most precisely measured in a whole-body calorimeter. The Atwater-Rosa-Benedict's human calorimeter has been used to verify the first law of thermodynamics in humans. · The metabolic rate can increase by a factor of 10-20 during steady state exercise. · Hepatic intermediary processes cause the specific dynamic activity (dietary thermogenesis) of proteins: Breakdown of amino acids, formation of urea etc. The dietary thermogenesis in general is related to mass action and temperature increase when eating. · Serious illnesses develop after a few weeks of fasting, because the cell structure proteins are broken down. The proteins of the cell nuclei produce uric acid, which accumulate in the heart (cardiac disease) and in the articulations (uric acid arthritis or podagra). · The lipostatic theory explains the constant body weight by liberation of a lipostatic, satiety peptide called leptin (ie, thin) from fat tissue. The plasma concentration of leptin is recorded by hypothalamic control centres, and seems to reflect the body fat percentage. Obese patients reduce their plasma leptin concentration by Banting and may lack the normal sensitivity to leptin. · The help of the body mass index (BMI) establishes obesity. BMI is the weight of the person in kg divided by the height (in m) squared. The normal range is 19-25 kg* m-2. Marginal overweight is defined as a BMI between 25 and 30 kg* m-2. Obesity is defined as BMI above 30 kg* m-2, which corresponds to body weights 20% above ideal weight. · Obesity results from a long-term excess of nutritional intake relative to the energy output. There are at least three types of obesity: genetic, over-eating and inactivity induced obesity. · Atherosclerosis, acute myocardial infarction, diabetes, hypertension, hypercholesterol-aemia, gall-stones, low concentrations of HDL, hyperuricaemia, gout, osteoarthritis of the hip and knee joint, and restrictive lung disease are all related to obesity. The Journal of Nutrition. Monthly journal published by the Am. Institute of Nutrition, 9650 Rockville Pike, Bethesda, MD 20814-3990, USA. Silverstone, T (1992) Appetite suppressants: A review. DRUGS 43: 820-836. Maffei, M et al. “Leptin levels in humans and rodents: Measurement of plasma leptin and of RNA in obese and weight-reduced subjects.” Nature Med 11: 1155-61, 1995. Katzung BG. Basic & Clinical Pharmacology. 11th Ed Appleton & Lange, Stanford, Connecticut, 2007.
|
||||||||||||||||||||||||||||||||
Click here to introduce your comments or contributions