New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 8:Cardiovascular Physiology & Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
|
|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
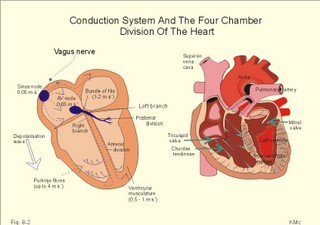
· To define afterload, anaemia, aneurysms, arterial pressure amplitude, diffusion- filtration- and permeability- coefficients, filtration capacity, preload, vascular compliance, and capillary protein permeability. · To describe the circulatory system, distribution of the total blood volume, capillary variability, capillary exchange-perfusion-permeability, venous system, venous pump, venous volume and pressures at different conditions. · To describe Laplace´s law, the law of conservation of matter for determination of volume and flow, the Starling equation, net filtration with lymph formation, oedema protection and formation, lymphatic oedema. · To draw a model of the paracapillary circuit and of the two circulatory systems. · To calculate one cardiovascular variable from selected variables given. · To indicate normal levels of cardiac output, oxygen uptake, arteriovenous oxygen content difference, oxygen binding capacity, haematocrit, haemoglobin concentration, mean corpuscular volume (MCV), mean corpuscular haemoglobin concentration (MCHC), and perfusion coefficients. · To explain the exchange between blood and cells, the control of erythropoiesis, Poiseuille´s law, total peripheral vascular resistance and organ resistance, vascular compliance and specific compliance, viscosity-related factors, and the Fåhræus-Lindquist effect. · To use the above concepts in problem solving and case histories. · The law of conservation of matter states that mass or energy can neither be created nor destroyed (the principle of mass balance). This principle is used to measure physiological blood volumes and bloodflow. · Poiseuille’s law is used both in the circulatory and the respiratory system (see Eq. 8-3). · Afterload is the force against which the ventricle contracts. A good index of the maximal afterload tension is the peak intraventricular pressure during systole. · Anaemia is defined as a clinical condition with an insufficient oxygen carrying capacity of the blood. A blood haemoglobin concentration below 130 g per l (8 mM) implies a measurable reduction of the working capacity for both sexes. · Arterial elastance or stiffness is (DPt/ DV) or the reciprocal of arterial compliance. · Arterial pulse amplitude or the pulse pressure is the difference between the systolic and the diastolic arterial pressure at a certain level. · Arteriovenous oxygen content difference is the difference between the oxygen concentration in arterial blood and that of the mixed venous blood (CaO2 – CvO2). · Bloodflow is the flow of whole blood to an organ per time unit. A practical index is the relative bloodflow measured per 100 g of tissue. Thus, the bloodflow is expressed in ml of blood per min per 100-g tissue, which is abbreviated as flow units (FU). · Bulk flow is convective transport of fluid with its content. · Capillary protein reflection coefficient (s) is the fraction of plasma protein molecules reflected off the capillary wall following collisions. · Cardiac output is the volume of blood leaving the left ventricle (or the right) each min. · Central venous pressure (CVP) is the pressure in the right atrium and caval veins close to the right atrium. · Compliance of a vessel is the increase of volume per unit of transmural pressure increase (DV/DPt). Transmural pressure refers to the intravascular pressure minus the extravascular pressure. · Contractility is a measure of the cardiac performance at a given preload and afterload. · Driving pressure is the mean arterial pressure minus the atrial pressure or CVP. · Ectopic focus is a pacemaker focus located in other regions of the myocardium than the sinus node. Active ectopic foci cause abnormal contraction patterns in the related regions of the heart. · Embolism refers to the process through which a thrombus is dislodged from its attachment and travels with the blood until it is lodged in a blood vessel too small to allow its passage. · Erythrocyte sedimentation rate (ESR) is the rate of fall of erythrocytes in a column of anticoagulated blood. ESR is increased, when the plasma is rich in large sticky protein molecules (fibrinogen, immunoglobulins etc) which agglutinate red cells, so they fall rapidly. Severe anaemia, immune reactions, infections, ischaemia, malignancy and trauma increases ESR. · Fibrinogen is a dissolved plasma protein that can be transferred to a blood cell trapping fibrin network by the proteolytic enzyme, thrombin. · Filtration: Transport across a barrier by means of a hydrostatic pressure gradient. · Haemolysis refers to disruption of the red cell membrane with liberation of the cellular content to the plasma of whole blood. · Haemostasis refers to the arrest of bleeding. · Hypocoagulability refers to a condition with a prolonged coagulation time. · Jaundice (icterus) is pigmentation of cell membranes, plasma and secretions with yellow bile pigments. · Mean arterial pressure (MAP) at a certain level equals diastolic pressure plus 1/3 of the pulse amplitude as an approximation. · Microcirculatory unit is a collection of vessels that originate from one arteriole, which is characterised by well-developed smooth musculature in its walls. · Oedema is an abnormal clinical state characterised by abnormal accumulation of interstitial or tissue fluid. · One atmosphere. By definition, one atmosphere equals 760 mmHg or 101.3 kPa. · Pinocytosis is a process by which fluid and large molecules can pass the capillary wall in vesicles formed by the cell membrane. · Preload is the end-diastolic filling pressure of the ventricle just before contraction. · Plasma viscosity is measured instead of erythrocyte sedimentation rate (ESR), because it is dependent of the same large protein molecules as ESR, but independent of haemoglobin concentration and obtainable within 15 min. · Pressure is force per area unit. The international unit is Newton per m2 or Pascal (Pa). · Pressure Resistance Units (PRU) are measured as Pascal seconds m-1 of blood (or as mmHg seconds ml-1 of blood). · Serum refers to plasma that has undergone coagulation and thus is devoid of fibrinogen and many other coagulation factors. · Serum ferritin concentration (Chapter 22) reflects the mass of stored iron in the body (normal range 12-140 nM). Most of the ferritin is stored in the tissues and not in the blood serum. · Serum iron concentration (Chapter 22) is Fe2+ bound to transferrin. The normal range is 7-36 mM with a mean value around 22 for both sexes. Iron deficiency leads to anaemia of the microcytic, hypochromic type (small, pale red cells). · Small-diameter phenomenon (Fåhræus-Lindquist): The viscosity of blood decreases in tubes with a diameter less than 0.5 mm, because the packed cell volume here is relatively low. · Solvent drag refers to transport of solvent, which can also draw solutes across a barrier. · Stroke volume is the volume of blood ejected from a heart ventricle with each beat. · Thrombosis refers to the formation of multiple thrombi or clots within the vascular system. · Total peripheral vascular resistance (TPVR) is the resistance of the systemic circulation. TPVR can be calculated as the driving pressure, divided by bloodflow (Q° s in ml per s): TPVR = DP/ Q° s. During exercise TPVR is reduced to approximately 30% of the level at rest. · Transferrin (Chapter 22) is a plasma protein vehicle with 2 binding sites for Fe2+ (normally 35% of the plasma globulin is saturated with iron). Transferrin saturation is the serum iron concentration divided by the total iron binding capacity. See iron deficiency. · Viscosity of blood is the inner friction, which is due to interaction between molecules and particles in the blood. The viscosity (h) one Pascal sec (1 Pa s) is the tangential force, working on 1 m2 of surface area, when dv/dx is 1 (s-1). This paragraph deals with 1. Circulatory organisation, 2. Haemopoiesis, 3. The red cells, 4. Viscosity, 5. Blood coagulation, 6. Vascular compliance and stiffness, 7. Wall tension, 8. Microcirculation, 9. Transcapillary fluid exchange and 10. The lymphatic system. General arrangement The cardiovascular system consists of two pumps arranged in series (Fig. 8-1). They are the right ventricle that pumps blood into the pulmonary circulation, and the left ventricle, which pumps blood into the systemic circulation. Each of these pumps delivers blood through an efferent tube system (the arteries) and each pump receives blood through an afferent tube system (the veins). In the pulmonary system, blood is pumped from the right ventricle through the lung capillaries and is temporary collected in the left atrium (Fig. 8-1). The coronary arteries are the first arterial branches that arise from aorta just above the aortic valve. Aorta and the elastic arteries are conductance vessels; the muscular arteries are distribution vessels; the arterioles are resistance vessels; the capillaries are exchange vessels; venules and veins are capacitance vessels. The arterio-venous anastomoses in fingers and toes are shunt vessels. Fig. 8-1: Design of human circulation with the right heart before and the left heart after the lungs. The principal function of the bloodflow in the cardiovascular system is to provide oxygen (O2) and nutrients to the tissues of the body and to remove carbon dioxide (CO2) and waste products. The flow of blood through the cardiovascular system follows physical law’s known from fluid mechanics (see principles). Strictly speaking, Poiseuille’s law (Eq. 8-3) has validity in a circulatory system, only when the fluid flow is laminar and non-pulsating in horizontally situated cylindrical vessels of constant dimensions. The resistance for laminar flow of a Newtonian fluid is only dependent on the dimensions of the vessel and the viscosity of the fluid. Resistance varies inversely as the fourth power of the radius of the vessel. For resistances in parallel, the total resistance is less than that of any individual resistance (Fig. 8-1 and Eq. 8-4). Although the total cross sectional area of all arterioles is much larger than that of all arteries, their resistance to bloodflow is much greater than that of the arteries. The number of daughter vessels is not high enough to balance the decrease in vessel diameter. The resistance is highest in the capillaries and it diminishes as the vessels increase in radius. For resistances in series, the total resistance equals the sum of the individual resistances (Eq. 8-5). In contrast to Poiseuille’s conditions, the bloodflow in the human circulation is pulsating and sometimes turbulent, and its blood vessels are not horizontally located, cylindrical or inflexible. Neither is the blood viscosity constant nor independent of vessel diameter and flow. At rest the mean red cell velocity in the capillaries is observed to be approximately 1 mm in one s; this provides ample time for gas exchange. Since the circulating blood moves continuously, the cardiac output must pass a cross section of all open capillaries. At rest a cardiac output of 5000 ml per min is a reasonable estimate; when changed into volume rate per s, the cardiac output is equal to 10-4 m3 s-1. Hence, it is possible to calculate the large cross sectional area of all open capillaries in a resting person according to Eq. 8-1 (see Fig. 8-1). The total blood volume is approximately 5 l in a healthy adult. The right atrium receives venous blood from the caval veins, and the left atrium receives oxygenated blood from the pulmonary veins. The two atria function as thin walled reservoirs and conduit organs for the blood (Fig. 8-2). On average, atrial systole contributes only about 15 % of the total ventricular filling, but in cardiac insufficiency the atrial contribution may increase importantly. The left and right ventricles provide most of the energy needed to transport the blood through the circulation. The left ventricle accelerates the blood into the systemic or peripheral high-pressure system, and its walls are thick in contrast to the thin, weak right ventricle, which pump blood into the low-pressure pulmonary system. The left ventricle consists of cardiac muscle fibres originating from the fibrous rings at the base of the heart and the fibres are twitching towards the apex. The orifice between the left atrium and the left ventricle carries two valve cusps, and this valve is called the bicuspid or mitral valve. Three cusps form the tricuspid valve closing the orifice between the right atrium and ventricle during systole. Strong filaments (chordae tendineae) arise from the papillary muscles of the ventricles. These chordae are attached to the free edges of the atrioventricular valves and normally prevent the valves from bulging into the atria during ventricular systole. The two atrio-ventricular valve systems prevent the leakage of blood backward from the ventricles into the atria (Fig. 8-2). Two other valve systems are interposed between the left ventricle and the aorta (the aortic valves) and between the pulmonary artery and the right ventricle (the pulmonary valves). The conduction system The normal heart is characterised by an electrical insulation between the atria and the ventricles mainly due to the fibrous ring (annulus fibrosus). However, the heart possesses a specialised electrical system, the cardiac conduction system that leads the electrical signal from the atria to the ventricles. The conducting system consists of modified myocardial cells. An optimal timing of atrial and ventricular pumping allows the emptying of the atria to be completed before the ventricular contraction. This allows the heart to pump the required cardiac output. The heart normally has a self-firing unit, located in the right atrium, called the sinoatrial node or sinus node (Fig. 8-2). The sinus node contains round cells (pacemaker cells), elongated intermediary cells and ordinary atrial cells. The electrical signal that automatically originates from the sinus node has the highest frequency, and the sinus node is thus the natural pacemaker of the heart. Even a cardiac transplant patient (the heart is totally denervated) adapts to the altered needs for cardiac function and of course initiates new heart beats as long as the transplant is functioning. The electric signal from the sinus node activates the atrial walls to contraction, and then reaches the main conduction system at the level of the atrioventricular node (AV node). The AV node consists of the same cell types as the sinus node. The impulse is delayed in the AV node, and this delay is allows the atrial systole to squeeze extra blood into the ventricles just before the ventricular systole occurs (see above). Fig. 8-2: The cardiac conduction system (left) is the only electrical connection between the atria and the ventricles of the normal heart. The anatomy of the four heart chambers is shown to the right. From the bundle of His, the signal is transmitted down a rapid conduction pathway, composed of the right and left bundle branches, to stimulate the right and the left ventricle and cause them to contract. The right bundle branch proceeds down the right side of the ventricular septum, and the large left bundle branch perforates the septum and divides into an anterior and a posterior division. These bundle branches divide into a network of conducting Purkinje fibres just below the endocardial surface. Purkinje fibres are large diameter cells without T-tubules, and with a long refractive period, so they can block premature depolarisation waves from the atria. The propagation wave spreads in the septum from both branches with the thick left bundle branch being dominant. The spread along the Purkinje fibres is rapid, whereas the spread from the endocardium to the epicardium is slow (Fig. 8-2). Ectopic foci become pacemakers, when the normal dominant pacemakers fail by blockade or depression: In the AV node, the atria, and the Purkinje fibres or in ischaemic ventricular fibres. These topics are developed further in Chapter 11. Distribution of blood and flow The total blood volume (5 l) is distributed with 60-75% in veins and venules, 20% in arteries and arterioles, and only 5% in capillaries at rest. Of the total blood volume only 12% are found in the pulmonary low-pressure system. The distribution of the cardiac output to the main organ systems of the body in a healthy person at rest and during maximal exercise is given in Table 8-1.
A top athlete can show a 6-fold increase in cardiac output from 5 to 30 l of blood each min, when going from rest to maximal dynamic exercise. The heart rate increases from 60 to 180-200 beats per min. The muscle bloodflow can rise from 3 to 75 ml per min per 100 g of muscle tissue (FU) or factor 25 in a total muscle mass of 35 kg. The muscular arterio-venous-O2 content difference can rise from the resting level (200 - 150) = 50 ml STPD per l of blood to (200 - 40) = 160 ml STPD per l. At rest the athlete typically has an oxygen uptake of 250 ml STPD per min. The total muscle bloodflow at rest is (35 000/100) ×3 = 1050 ml of blood per min. The total muscular oxygen uptake at rest is (1050* 50/1000) = 53 ml per min (Table 8-1). During maximal dynamic activity the total muscle bloodflow is: (35 000/100)×75 = 26 250 ml/min or 26.25 l per min. The total muscular oxygen uptake is increased to (160 × 26.25 l per min) = 4200 ml STPD per min (Table 8-1). Accordingly, the total muscular oxygen uptake rises by a factor of (4200/53) almost 80 from rest to exercise. At the start of exercise, signals from the brain and from the working muscles bombard the cardiopulmonary control centres in the brainstem (see Chapter 18). Both cardiac output and ventilation increase, the a-adrenergic tone of the muscular arterioles falls abruptly, whereas the vascular resistance increases in inactive tissues. The systolic blood pressure increases, whereas the MAP only rises minimally during dynamic exercise. The total peripheral vascular resistance (TPVR) falls during exercise towards 30% of the level at rest, because of the massive vasodilatation in the muscular arterioles of almost 35 kg muscle mass (Eq. 8-3). This is why the major portion of the cardiac output passes through the skeletal muscles (Fig. 18-1) and why the diastolic pressure often decreases during exercise. At moderate exercise the skin bloodflow and heat dissipation is increased (Chapter 21). The coronary bloodflow increases from rest to exercise (Fig. 10-7 A to B). Haemopoiesis is the formation of blood cells. All blood cells are derived from the multipotent stem cells. Stem cells produce erythroid cells, granulocytes, lymphoid cells, megacaryocytes and monocytes by a number of differentiation steps. Stem cells maintain normal cell populations in a healthy bone marrow controlled by haemopoietic growth factors, and stem cells have the capacity for self-renewal. Haemopoietic growth factors include erythropoietin, interleukins, glucocorticoids, sex hormones and thyroid hormones. Stem cells and red cell precursors contain ribosomal RNA along with cell organels. The cells lose organels during maturition. Pronormoblasts, normoblasts and reticulocytes at each stage contain less RNA and increasing amounts of haemoglobin. Reticulocytes can still synthesise haemoglobin, have lost the nucleus, and remain in the bone marrow a few days before they enter the peripheral blood. Here, they lose their RNA after a couple of days and become mature red cells. The reticulocyte count is normally less than 2.5% of the red cell count, but following haemorrhage or haemolysis the reticulocyte-% increases reflecting increased erythropoiesis. When the bone marrow fails to respond to anaemia, the reticulocyte count may fall below 0.5%. The normal haematological ranges are given in Table 8-2, together with other values of interest.
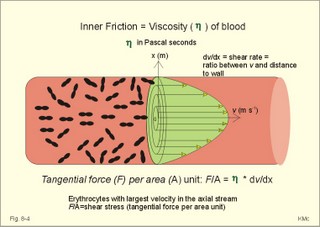
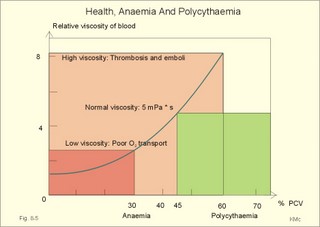
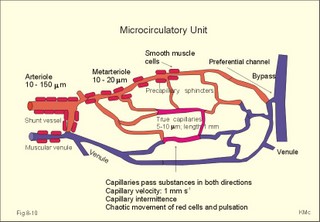
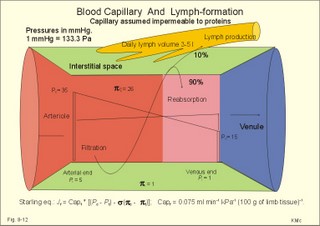
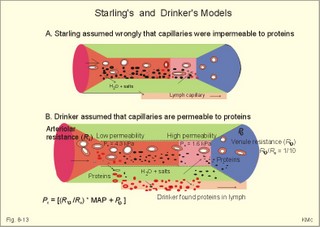
When normal kidneys are perfused with hypoxaemic blood, the peritubular interstitial cells release large amounts of the glycoprotein hormone, erythropoietin, with a strong effect on the haemopoietic stem cells in the red bone marrow. The stem cells are stimulated to produce proerythroblasts, which speed up the production of new red cells after a few days. The increased erythrogenesis improves tissue oxygenation, which decreases erythropoietin production and the balance is re-established. Chronic renal failure leads to erythropoietin deficiency, and thus to anaemia, which is of the normochromic, normocytic type. Haemoglobin is synthesised in the mitochondria of the maturing red cells. Vitamin B6 is a co-enzyme for the formation of d-amino-laevulinic acid (ALA) by ALA-synthetase. The reaction is stimulated by erythropoietin. One haemoglobin molecule binds 4 oxygen molecules at most. Haemoglobin consists of globin (2 a and 2 b polypeptide chains) and 4 prostetic haem-groups (Fig. 8-3). Haemoglobin A (for Adult) has a molecular weight of 64 460 g per mol (Dalton). Haemoglobin A comprises almost all haemoglobin in adults, supplied with only a minimum of haemoglobin A2. The polypeptide chains are not covalently linked but are held together by hydrophobic forces. Each haem group is connected to one polypeptide chain, which contain a ring of 4 imidazol-groups. In the centre of the porphyrin ring the one iron atom is coordinated by 6 ligands, four of which bind the metal to the porphyrin chain, one to histidin on either the a- or the b-chains. The last is an open binding, which is able to bind either O2 or carbon monoxide (CO). In the lung capillaries haemoglobin is saturated with oxygen at high tensions, where the affinity of (oxy)haemoglobin for more oxygen is high (Fig. 8-3). The affinity between oxygen and haemoglobin is defined and described in Chapter 15, where P50 is introduced as an affinity index. A low P50 equals a high standard affinity and vice versa. The successive change in affinity during binding of the 4 oxygen molecules to each haemoglobin is caused by molecular interactions among the 4 haem groups. This explains the sigmoid shape of the oxygen dissociation curve (Fig. 15-3). Oxygen is released at the low tensions of the tissues, where the affinity of (deoxy)haemoglobin for oxygen is low. The oxygen tension in the tissue mitochondria may reach extremely low values (zero to 1 mmHg or 0.133 kPa). Red cells do not contain mitochondria, so they survive on anaerobic metabolism (glycolysis) and the anaerobic intermediate, 2,3-diphosphoglycerate (2,3-DPG), is produced by the help of a red cell enzyme. As the 4 haem units successively unload oxygen, the b-chains of deoxyhaemoglobin are pulled apart, and 2,3-DPG binds strongly to the 2 b-chains of deoxyhaemoglobin (Fig. 8-3). This electrostatic binding substantially reduces the affinity between oxygen and haemoglobin. – Individuals with high arterial pH (chronic alkalosis) or with low arterial oxygen tension (hypotonic hypoxaemia) increase their concentration of 2,3-DPG in their red cells. Storage of blood reduces the 2,3-DPG concentration with time. Fig. 8-3: Model of oxyhaemoglobin (relaxed binding structure) and deoxyhaemoglobin (tight binding structure). The circular disc with Fe is haem. When haem is bound to O2 or CO, it has a cherry-red colour, and haem is dark red when it is in the deoxygenated form. The breakdown of haemoglobin liberates CO and produces bilirubin that is yellow in colour. Bilirubin is normally excreted with the bile. Failure of bile excretion leads to accumulation of bilirubin in the body. Jaundice (icterus) is a yellow pigmentation of the skin, plasma, cell membranes and secretions with accumulated bilirubin and other bile-pigments. Bilirubin and other pigments are also found in the blue-yellow skin-spots following lesions with subcutaneous bleeding. Notice that when blood is saturated under the normal, ambient O2 partial pressure (20 kPa = 150 mmHg), the oxygen capacity of haemoglobin is 1.34 and not 1.39 ml STPD g-1 (Fig. 8-3). The latter holds only for extremely high partial pressures (above 45 kPa), when breathing pure oxygen or oxygen enriched air, where the oxygen capacity is equal to the theoretical. The rate of fall of red cells is called the erythrocyte sedimentation rate (ERS). The ERS is measured in a glass column of whole blood with anticoagulant. ERS is measured in mm as the cell free yellow zone above the red cells following 60 min of sedimentation. ERS is an estimate of the acute phase response. The acute phase response produces high levels of large sticky proteins (C-reactive protein, immunoglobulins, fibrinogen) that form rapidly falling piles of red cells. ERS is abnormally increased (above 20 mm) in infections, immunology reactions, ischaemia, malignancy or traumas. Normally, the level is only a few mm per first hour, 15-20 with a common cold, and 50-100 during pregnancy. Viscosity is the inner friction in the fluid, which is due to the interaction between molecules and particles in the blood passing a cylindrical vessel. Telescope cylinders (laminae) of blood sliding against each other (Fig. 8-4) can illustrate this inner friction. The outermost blood cylinder rests against the vessel wall (velocity is zero), and the central cylinder moves (laminar flow) with the greatest velocity (v). The velocity profile is parabolic. The velocity gradient, with the distance x from the centre of the blood vessel towards the outermost blood cylinder, is called the shear rate (dv/dx). The tangential force (F) between these blood cylinders depends upon the area (A) sliding against each other, and the relation to viscosity (h) is given by the equation in the legend to Fig 8-4. Fig. 8-4: Blood vessel with red cells and arrows shoving different velocity (v). F/A = h × dv/dx. The viscosity (h) one Pascal sec (1 Pa s) is the tangential force, working on 1 m2 of surface area, when dv/dx is 1 (s-1). This simplified description is valid for water, gas, and other homogenous fluids that are Newtonian fluids. Newtonian fluids are defined as fluids with a viscosity that is independent of the shear rate. Newtonian fluids move streamline or with so-called ideal laminar flow. The viscosity of non-Newtonian fluids decreases with increasing shear rate, according to the equation above. Blood is namely not homogenous with a viscosity that is independent of shear rate. On the contrary, at low shear rates (low bloodflow), the viscosity of blood can be ten-fold higher than normal. The typical normal viscosity of body warm blood is 5 centiPoise equal to 5 milli-Pascal seconds or 5 (mPa*s). Blood viscosity depends upon the concentration of red cells (the haematocrit). Fig 8-5: Haematocrit (PCV) and relative viscosity varies along the green line. A normal PCV of 45% is shown with the normal absolute viscosity of body-warm blood. A patient with anaemia and a PCV of 30% has a low blood viscosity and a poor oxygen transport capacity (Fig. 8-5). On the contrary, a patient with polycythaemia and a PCV of 60% has a high oxygen transport capacity, but the blood viscosity is dangerously high and he may develop thrombosis and emboli (Fig. 8-5). With increasing bloodflow (and shear rate), an increasing fraction of red cells is being pulled into the axial stream of small vessels, so that friction is being minimised. At high shear rates in large vessels, blood therefore mainly behaves like a Newtonian fluid, with a low and almost constant viscosity, as well as a linear relation between bloodflow and the driving pressure. The viscosity of blood apparently decreases in tubes with a diameter less than 0.5 mm (the small-diameter effect - or the Fåhraeus-Lindqvist phenomenon - see Fig. 8-6). Fig. 8-6: The viscosity of blood decreases abruptly in tubes with diameters decreasing from 0.5 mm (Fåhræus-Lindquist effect). This is because the packed cell volume (PCV) is low in small vessels, since red cells have a tendency to accumulate and pass as a single plug in the fast axial stream, where there is a negligible friction. The slower layers along the vessel wall are passed mainly by plasma. This falling viscosity in the small resistance vessels and in the precapillaries and capillaries reduces the work of the heart. This is why the bloodflow frequently rises linearly with the driving pressure and thus actually follows Poiseuille´s law, as if blood was a Newtonian fluid. Bloodflow tends to become turbulent in irregular vessels, where the flow velocity is high and the viscosity is low. Turbulence means irregular movements of the fluid elements - an energy demanding transport process. Plasma viscosity is sometimes measured instead of erythrocyte sedimentation rate (ESR), because it is dependent of the same large sticky protein molecules as ESR, but is independent of the haemoglobin concentration and obtainable within 15-20 min. Whole blood consists of a fluid (plasma) in which blood cells and platelets are suspended. Blood cells consist of red cells (erythrocytes) and white cells (leukocytes). A small amount of anticoagulant to a blood sample blocks the coagulation process, and whole blood sediments into three layers: Below the heavy red cells, then a thin grey-white layer of white cells, and above a yellow fluid (plasma) with an invisible content of most of the platelets. A blood sample without anticoagulants normally sediments with coagulation (fibrin formation) within 5 min. A firm red mass is formed, and after some time it retracts and forms a red cone (a fibrin clot of blood cells and fibrin) surrounded by yellow serum. Healthy humans possess both a fast extrinsic and a slow intrinsic clotting system. The coagulation process involves at least 3 systems all contributing to the haemostasis. Firstly, a vasoconstriction occurs following release of serotonin from damaged endothel cells. Secondly, the fast extrinsic system goes into action, and thirdly, the slow intrinsic system contribute. Finally, the 2 coagulation systems operate together and converge for common reactive steps in order to produce thrombin (Fig. 8-7). Disruption of the endothelial barrier by injury initiates a cascade of catalytic events through either or both clotting systems. At each reaction in the chain of events, a proenzyme coagulation factor is activated to its enzymatic form, which can activate the next reaction in the chain. The letter a stands for the active form. The enzymes are all endopeptidases (proteases), and their catalytic sites include a serine moiety. By these many steps in the cascade, the process escalates until large amounts of thrombi are released. - Factor IV (Ca2+), factor V (proaccelerin), kininogen, kallikrein, and factor VIII are coagulation co-factors without enzymatic activity. Thrombin is a protease that is responsible for the formation of fibrin monomers, and thus for formation of a fibrin clot. Its parent molecule is prothrombin (factor II), which is present in normal plasma. Thrombin formation from prothrombin goes through certain cleavage stages, the first of which is by activated factor Xa (Stuart). These reactions are augmented by factor IV (Ca2+), factor V (proaccelerin), and phospholipid (see green oval in Fig. 8-7). Thrombin initiates blood platelet aggregation, and disintegrates the plasma membrane of the platelets so phospholipid is provided. The coagulation factors are synthesised mainly in the liver. - An exception is the large Von Willebrands factor (vWf) complex, which is synthesised in the vascular endothelial cells and in megakaryocytes. The fast extrinsic thrombin formation is initiated by the contact of blood with injured cells (Fig. 8-7). The damaged cells liberate a clot-promoting agent, factor III or tissue thromboplastin. Factor III interacts with a plasma protein, factor VII, to start a cascade of reactions by prothrombin activators leading to formation of thrombin within seconds (Fig. 8-7). Clotting of blood implies conversion of a soluble plasma protein, factor I (or fibrinogen), into an insoluble network of fibrin. First, fibrinogen undergoes limited proteolysis by thrombin. The formed fibrin monomers polymerise into insoluble strands of fibrin polymers (Fig. 8-7). Finally the monomers of the fibrin strands are cross-linked by the enzyme activated (a) fibrin-stabilising factor (XIIIa). Fig. 8-7: Blood coagulation and fibrinolysis. The roman numbers were originally introduced as a short-cut. When venous blood is drawn in silicone coated tubes and centrifuged for the separation of cells and plasma, the isolated plasma clots readily, due to the negative surface charge of glass. In the absence of thromboplastin, thrombin is formed via the intrinsic clotting system. Negatively charged surfaces on damaged cells generate thrombin, trigger inflammatory and immune responses and even activate fibrinolysis. The first step is that negatively charged surfaces (artificial or injured endothelial barrier) activate factor XII (Hageman) to XIIa, which can activate factor XI in the presence of kininogen. The factor XIa activates the vitamin-K-dependent protein, Christmas factor (IX). Christmas factor is synthesised under the control of a gene on the X-chromosome. Activated Christmas factor (IXa) converts factor X to its activated state (Stuart factor Xa). Stuart factor is a plasma proenzyme - also vitamin-K-dependent. The Xa is the enzyme immediately responsible for the release of thrombin, and the final steps of the two clotting systems are identical (Fig. 8-7). Hepatocytes produce factors X, IX, VII, and II only when vitamin K is present. Insufficient synthesis of these coagulation factors can lead to serious bleeding. When the endothelial surface of the vascular system is disrupted, platelets normally adhere instantly to exposed structures (collagen and other fibres). Adherent platelets discharge ADP and other substances. Adherent platelets become spherical and send out spicules that look like the legs of a spider. The platelet plug grows and forms a firm haemostatic plug that stops the bleeding. Platelets provide substances that enhance thrombin production, such as phospholipid, the important cofactor in the clotting process. Blood has the ability to dissolve clots. Fibrinolysis is the dissolution of fibrin. The hepatic plasma glycoprotein proenzyme, plasminogen, is activated to the serine protease, plasmin (Fig. 8-6). Streptokinase, staphylokinase and urokinase convert plasminogen to plasmin. The tissue plasminogen activators are serine proteases. Stress, muscular activity and emotional crises enhance fibrinolysis. Plasmin digests fibrin, fibrinogen and other clotting factors. If plasmin is formed in blood plasma devoid of clots, it is irreversibly inhibited by a2-antiplasmin (Fig 8-7). The coagulation process is normally modulated to the needs of the person by inhibitors within the blood. Antithrombin III is the main inhibitor of thrombin and factor Xa, and its effect is potentiated by heparin. Heparin is a negatively charged mucopolysaccharide from mast cells. Heparin binds to antithrombin III forming a complex that rapidly binds serine proteases such as thrombin, thus functioning as a potent anticoagulant. Heparin alone does not inhibit the coagulation process significantly. Heparin is used during artificial perfusion such as dialysis and open heart surgery. Exogenous plasminogen activators are used to dissolve clots in the coronary arteries. Fibrinolysis is inhibited mainly by a2-antiplasmin, because plasmin combines with antiplasmin in an irreversible link (Fig 8-7). Vitamin C or ascorbic acid cannot be synthesised in humans, but the vitamin is present in all fresh fruit and vegetables. Hydroxylation of proline to hydroxyproline is necessary for the formation of collagen and thus of the normal tissue including blood vessels. Lack of vitamin C (scurvy) leads to defective blood vessel walls with spontaneous haemorrhage and blue spots. 6. Vascular compliance and stiffness Distensibility or compliance is the increase of volume per unit of transmural pressure increase (DV/DPt). The specific compliance is the relative increase in volume per unit of pressure increase. The elastance or stiffness is the reciprocal value of the compliance. The compliance of the venous system can be 30 times as large as that of the arterial system. The venous system can be expanded to contain more than 75% of the total blood volume. The veins function as capacitance vessels, and become very distended when blood is given in transfusions, in heart insufficiency, or during a heart attack. Severe exercise and loss of blood cause an increase in venous tone, which for a period actually can increase the circulating blood volume. During hard work the muscular venous pump provides up to 1/3 of the energy required for blood circulation (the peripheral venous heart). The venous system also plays an important role by its graded venous return to the heart. Fig. 8-8: Recording of the arterial blood pressure at rest. Dashed, horizontal lines depict MAP. The pulse pressure of the abdominal aorta is calculated from the arterial compliance of a young person at rest. The recording shows a systolic peak pressure, a dicrotic notch as the aortic valves close, and a falling diastolic pressure. The yellow area under the dashed green line equals the yellow area above the line (Fig. 8-8). The mean arterial pressure, MAP, is usually being defined as the diastolic pressure plus 1/3 of the pulse pressure (Fig. 8-8). The mean arterial pressure (MAP) is about 12 kPa (= 90 mmHg) in the arteries. Notice the fall in MAP from the abdominal aorta to the femoral artery, whereas the systolic pressure increases. The arterial mean pressure falls to a mean value around 2.4 kPa (18 mmHg) in the capillaries. The arterial pulse pressure is the difference between the systolic and the diastolic arterial pressure. At a heart rate of 75 beats/min at rest, the cardiac cycle length is 0.8 s with 0.3 s systole and 0.5 s diastole. A stroke volume of 70 ml is deposited in the aorta and the larger elastic arteries during systole. During the systolic period 26 ml of blood (70×3/8) is streaming through the resistance vessels, leaving the arterial system, so the systolic volume expansion is 44 ml of blood. A young healthy subject has an arterial distensibility or compliance of 1 ml of blood per mmHg, which creates a pressure rise during systole (pulse amplitude) of (70-26) = 44 mmHg (Fig. 8-8). With a diastolic pressure of 70 mmHg, this implies a systolic pressure of 114 mmHg, conventionally written 114/70 mmHg or 15.2/9.3 kPa. Aging and arteriosclerosis increase the stiffness (reduce the distensibility) of the elastic arteries, causing the arterial compliance to fall from 1 (one) to 0.5 ml of blood per mmHg. In this case, a systolic volume expansion of 44 ml of blood increases the pulse pressure amplitude to 88 mmHg (44/0.5=88), and the blood pressure to perhaps 180/92 mmHg. This is a likely process in an otherwise healthy person of advanced age. Typically, the average diastolic pressure will rise with age. For a thin-walled organ with two main radii, Laplace predicted that thetransmural pressure at equilibrium (DPt), was identical with the fibre tension in the wall (T) divided by the two main radii: DP = T /(r1 + r2). This model has often been used (withmodifications for wall thickness, w) for the relaxed ventricle (Fig. 8-9A). Fig. 8-9: Laplace models for the relaxed ventricle (A), the spherical alveole (B), and the cylindrical blood capillary (C). For a thin-walled spherical organ (r1 = r2 = r), another Laplace equation can be developed from the equation above (Fig. 8-9B). This model is often used for both alveoli and the spherical ventricle. When the left ventricle becomes more and more spherical by diastolic filling, the T will rise with the transmural pressure. The radius increases with the end-diastolic volume. The more the end-diastolic pressure and the fibre tension rises, the higher is the energy demand and the more O2 is consumed per heart beat during contraction of the dilatated ventricle. For an infinitely long thin-walled cylinder, like a true capillary or a preferential channel (see below), the r2 approaches infinity and has no influence on the transmural pressure. Hence, the Laplace equation can be approximated by eq.C in Fig. 8-9C. This model is used for a thin vessel wall, since T/r2 approaches zero. Surprisingly enough, the thin endothelial barrier (0.3 mm) of a capillary easily carries a pressure of 4.3 kPa (32 mmHg) or more. This is because the capillary radius is so small. According to eq. C in Fig. 8-9C, a small radius (5-10 mm) must imply a small wall tension (T). In hypertension, the arterial walls hypertrophy, so the wall tension is minimised and hence the risk of vessel rupture (use Eq. 8-9C with correction for wall thickness: T = DP* r/w). The microcirculation is responsible for the transport of nutrients and oxygen to the tissues, and for removal of cellular waste products and CO2. The arterioles control the flow of blood to each tissue unit, and the metabolic conditions of the tissue cells determine the diameters of the vessels. Hereby, the tissue unit often controls its own blood flow by local mechanisms. A microcirculatory unit is a collection of vessels that originate from an arteriole, which is characterised by well-developed smooth musculature in its wall (Fig.8-10). Arterioles of the face, fingers and toes often branches into an arteriovenous anastomose, which functions as a shunt vessel, but which also can be closed completely. In certain tissues the arteriole branches into metarterioles (with so-called precapillary sphincters of smooth muscle fibres without nervous supply), which continue into large capillaries termed preferential channels (or thoroughfare channels). These channels shunt the blood to the veins. The small true capillaries have only a thin endothelial cell layer making the wall ideal for exchange Fig. 8-10: A microcirculatory unit. The diameter of true capillaries is only 5-10 mm, barely enough for erythrocytes to squeeze through. The average length of capillaries is 1 mm, and the linear red cell velocity at rest varies around 1 mm each s. The capillary density is high in cardiac and striated muscle tissue and low in subcutis and in cartilage. Endothelial cells contain actin and myosin. It is uncertain whether capillaries may be able to alter their shape according to the needs of the tissues. Important exchange vessels are thin-walled vessels with a large surface area. Exchange vessels comprise true capillaries, parts of preferential channels, and venules (Fig. 8-10). The number of pores is high in the venous ends of capillaries and in venules. Exchange vessels are any blood vessels, which allow transport of substances through its wall in both directions. The velocity of the bloodflow in capillaries varies, sometimes with rhythmic pulsation, at other times random. At rest the intracapillary pressure varies from arteriole to venule between 3.3 and 1.6 kPa (25 and 12 mmHg), during arteriolar vasoconstriction between 1.6 and 1 kPa (12 and 8 mmHg), and during vasodilatation between 5.3 and 1.6 kPa (40 and 25 mmHg). Arterial pressure fluctuations have been recorded even in the most distal parts of the capillaries. In venules and veins, however, the flow is smooth without fluctuations. The capillary wall consists of a layer of endothelial cells (0.1 - 1 mm of thickness) resting on a basement membrane. At least three types of capillaries are present in humans: 1. Continuous capillaries are the most abundant. The distance between endothelial cells is 5-30 nm (Fig. 8-11). Tight junctions with narrow clefts are difficult to pass for the dissolved molecules and ions. In the continuous capillaries, the water filled pore surface area comprises only 10-4 of the total surface. The continuous capillaries in the brain are low permeable to ions and most hydrophilic molecules, because their tight junctions are really tight (the blood-brain barrier). 2. Fenestrated capillaries contain tight junctions and pores or fenestrations, which are fluid filled channels with a diameter of 50-100 nm. These are formed by two adjacent cell membranes that have fused during removal of the lipid bilayers, so only a diaphragm of protein lattice is left allowing bulk flow without colloids (Fig. 8-11). Fenestrations are round windows found in the capillaries of organs that transport lots of water (the bowels, glomerular capillaries of the kidneys, pancreas and salivary glands). In each fenestration a bush-like filaments can be demonstrated by electron micrography (Rostgaard). The filaments are composed of a protein core with glycosaminoglycan side chains. The filaments and the protein lattice in the fenestrae keep plasma proteins back (Fig. 8-11). In the glomerular capillaries, water filled fenestrations cover 20% of the surface. Fig. 8-11: Three types of capillary walls. 3. Sinusoid capillaries have very broad openings between the endothelial cells (Fig. 8-11). These large fenestrations have no diaphragm. Sinusoid capillaries are often found in tissues that are bathed in plasma (liver, spleen and bone marrow). The circumventricular organs of the brain contain lots of fenestrations in the walls. The circumventricular organs are located close to the control centres of the hypothalamus and the brainstem. Any penetration of signal molecules in the neighbourhood of these control centres is of physiological importance. - In other areas with continuous capillaries, most substances cannot bypass the blood-brain barrier and reach the brain cells. 9. Transcapillary fluid exchange Starling hypothesised that the fluid exchange across the capillary wall was determined by the hydrostatic (Pc) and the colloid osmotic pressure (pc) in the capillary (Fig. 8-12). Fig. 8-12: Transcapillary fluid exchange (Starling) is shown over a capillary wall. The pressures are in mmHg. The capillary filtration coefficient is in ml*min-1*kPa-1*(100 g tissue)-1. One mmHg equals 133.3 Pa. The flux of substance (J) over the capillary membrane is determined by (P × DC). Actually, this is nothing but an extension of Fick’s law for diffusive transport (Eq. 1-2). Fluid moves out of the arterial end of the capillary by filtration, because the net hydrostatic pressure (35-5 = 30 mmHg) is higher than the colloid osmotic pressure (pc= 26 mmHg), and most of the fluid (9/10) passes again into the blood by reabsorption in the venous end (Fig. 8-12). Here, the colloid osmotic pressure (26 mmHg) supersedes the hydrostatic pressure (15-1 mmHg equals14 or 1.9 kPa). The net diffusion of water molecules across the capillary wall is approximately zero. Instead, the transvascular exchange is caused by a combination of an outward ultrafiltration and an inward colloid osmotic force. Ultrafiltration is caused by a hydrostatic pressure gradient created by the heart. The hydrostatic pressure gradient is a net outward force, moving water through pores in the capillary wall. Plasma contains dissolved protein, which cannot pass the small pores in capillary walls readily. The plasma proteins create a colloid osmotic pressure of about 3.3-3.7 kPa (25-28 mmHg). This pressure is much larger than the interstitial colloid osmotic pressure, so that the colloid osmotic gradient across the capillary wall is a net inward force, which draws water into the capillaries. Starling in Eq. 8-7 described the transvascular water flow as early as in 1896. The driving forces are the so-called Starling forces (see Eq. 8-7). The capillary protein reflection coefficient is symbolized s. s is the fraction of plasma protein molecules reflected off the capillary wall. The protein reflection coefficient is 0.9-1.0 for many capillaries, expressing that the colloid osmotic pressure gradient is not reduced over time by diffusion of proteins over the capillary wall. The capillary filtration coefficient (Capf) corresponds to the permeability of the capillary wall. In the legs Capf is around 0.075 ml of fluid per min per kPa in 100 g of tissue (at body temperature). The combined pressures in the Starling equation ([(Pc - Pt) - s(pc - pt)]) determine, if there is a net pressure for water movement across the capillary wall (Eq. 8-7). In conclusion, water moves out of the arterial end of the capillary by filtration, and near the venule end, water moves into the blood by reabsorption. This transport along the capillary is called Starling´s paracapillary circulation. Thus there is normally a net filtration of water and some proteins into the interstitial space. This water and protein, returns to the blood via the lymphatic system (1/10 of the total filtration in Fig. 8-12). The lymph volume amounts to approximately 3-5 l daily, and is mainly produced in the liver and intestine. Starling presumed – erroneously - that proteins were unable to leave the blood in the capillaries (Fig. 8-13: A). Fig. 8-13: Two models of transcapillary fluid exchange. The capillary pressure (Pc) is protected from large changes in MAP, but is sensitive to changes of venous pressure including the central venous pressure. This assumption is wrong. The capillaries are almost universally permeable to proteins and macromolecules that resemble proteins. Another physiologist Drinker found protein in lymphatic fluid. Drinker developed a model, which presumed that capillaries to a variable degree were permeable to proteins (Fig. 8-13: B). Within a single capillary, the protein permeability increases from the arterial towards the venous end. Let us assume that the heart is pumping out about 9000 l of blood every day. With a packed cell volume of 45% there is 55% plasma. This means that 4950 l is plasma. With a 6% protein concentration there is a total of 297 kg of protein. If less than 0.1 per cent (1/1440) of this protein is filtered into the interstitial fluid and lymph, it amounts to 206 g of protein daily. This amount of protein leaves the blood in the capillaries, and returns almost completely to the blood through the lymph and not the veins (Fig. 8-13: B). Hence, Starling’s paracapillary circulation obviously plays a dominating role in the transport of crystalloids (small molecules of nourishment and waste products) through the capillary wall. The capillary hydrostatic pressure (Pc) varies from tissue to tissue. It is low in the lungs and intestine (1 kPa) and particularly high in the renal glomerular capillaries (6-8 kPa). In resting skeletal muscle capillaries, the pressure is 4.3 kPa (32 mmHg) at the arterial end and 1.6 kPa (12 mmHg) at the venous end. In general, Pc increases whenever the mean arterial pressure (MAP) increases, venule pressure (Pv) or resistance (Rv) increases, or when arteriolar resistance (Ra) decreases, according to the formula: Pc = [(Rv/Ra) MAP + Pv] developed in Fig. 8-13. Normally, Rv/Ra is approximately 1/10. Thus Pc is protected from large changes in MAP, but is sensitive to changes in venous pressure including the central venous pressure (CVP). In tissues, where the perfusion pressure is reduced to a value below a so-called critical closing pressure, the bloodflow ceases due to vessel collapse. This is explained by the Laplace model (Fig. 8-9C). The myogenic response also causes an important deviation from Poisseuille´s law. The myogenic response covers reactions where the vascular smooth muscle contracts in response to increased transmural pressure and vice versa. A decrease in transmural pressure (intravascular minus extravascular pressure) of the precapillary vessels elicits precapillary relaxation. A rise in transmural pressure elicits precapillary contraction. Perhaps the stretch of smooth muscle cells opens Ca2+-channels, whereby a Ca2+-influx increases the intracellular Ca2+ concentration sufficiently for contraction. Macromolecules do penetrate the capillary wall and the content of lymph derives from plasma. Less than 0.1 per cent of all the plasma proteins that are being ejected from the heart in 24 hours, escapes from the capillaries. The venous end of the capillaries is permeated by pores of 40 - 60 nm. Here, macromolecules can pass by filtration in a pressure determined fluid transport. Passage as a whole plasma portion (bulk flow) through fenestrations is also possible. Transepithelial solvent transport can also draw solutes by solvent drag. Gradient dependent transport concepts such as filtration, bulk flow and solvent drag are used by different groups of scientists. When large amounts of lymph is being produced, solvent drag dominates over diffusion. At low lymph production, half of the protein transport is caused by diffusion. Fluid pass through the cell by pinocytosis. Capillary filtration predominates over capillary reabsorption resulting in an overshoot (a net filtration) of interstitial fluid. Most of the net filtration is reabsorbed into the blood of end-capillaries or venules (Starling´s paracapillary circulation). The lymphatic vessels drain the remaining filtered fluid (Fig. 8-12). The lymphatics are composed of endothelium-lined vessels similar to blood capillaries. Some lymphatics are equipped with one-way valves, so rhythmic activity in nearby skeletal muscles returns the lymph to the circulation via the thoracic duct. Lymph vessels originate as blind-ended sacs close to the blood capillaries. Lymph vessels are permeable to proteins, macromolecules and even to cells from the interstitial fluid. The lymphatic drainage is particularly important for transporting chylomicrons absorbed from the intestine, and to return plasma proteins that leaks from several blood capillary systems. Lung tissue has no lymphatics, because the lymphatic vessels end at the terminal bronchioli. The lymph from the liver provides us with 50% of the daily lymph produced. Lymphatic fluids from liver and kidney have a protein concentration equal to plasma’s (6-8 g per 100 ml), and lymphatic fluid from the bronchial tree has a similar concentration of protein. Lymphatic fluids from skin and muscles contain only 2% protein, and brain lymph contains no protein at all. This paragraph deals with 1. Anaemia, 2. Oedema, 3. Thrombosis/Embolism, 4. Haemophilia 5. Aneurysms and 6. Valvular diseases. Anaemia is defined as a condition with an insufficient oxygen carrying capacity of the patients blood. For both sexes and all age groups a blood haemoglobin concentration below 130 g per l (8 mM) implies reduced working capacity and thus a consequential clinical condition. Reference levels for age and sex are also available, but they differ from laboratory to laboratory. Mean corpuscular volume (MCV) expresses the mean volume of each red cell. MCV is calculated from the packed cell volume (PCV) by division with the red cell count. An example with normal values provides the following: 0.45 (l/l)/5*1012 (red cells/l). Thus MCV is equal to 90*10-15 l per red cell. One femtolitre (1 fl) equals 10-15 l. The normal range is 80-96 fl. The MCV index is used to classify anaemia’s into microcytic (MCV<80 fl), normocytic (MCV 80-96 fl) and macrocytic forms (MCV >96 fl), but the classification is not causal. Mean corpuscular haemoglobin concentration (MCHC) provides the mean concentration in each red cell. MCHC is calculated from the haemoglobin concentration by division with the packed cell volume (PCV). An example with normal values provides the following: 150 (g/l)/ 0.45 (l/l). Thus, normal MCHC is 333 g per l of red cells. Since the concentration of haemoglobin in a normal red cell is maximal, the maximal value (380 g/l) is the highest occurring. Normochromic anaemia’s have MCHC values in the range 320-380 mostly within 320-350 g/l. Anaemia with MCHC below 320 g/l is called hypochromic, and they are often also microcytic such as in iron deficiency anaemia. Anaemias are classified into two groups based on their cause. The first group is deficiency anaemias with insufficient haemoglobin production due to dietary/ absorptive defects or to bone marrow hypoplasia from cell destruction by chemicals or radiation (Table 8-3). Deficiency anaemias are caused by defect haem synthesis (iron deficiency, anaemia of chronic disease, sideroblastic anaemia) or by defect globin synthesis (thalassaemia). The second group is waste anaemias with waste of red cells (Table 8-3). The waste of red cells is caused by bleeding (haemorrhage) or by haemolysis.
A1. Iron deficiency anaemia is caused by chronic bleeding, growth, endurance exercise, pregnancy and nursing, poor intake, malabsorption). Iron deficiency is characterised by low serum-iron, high total iron binding capacity (TIBC), and a transferrin saturation below 19%. A2. Anaemia of chronic disease (defect synthesis of haem): 1.Chronic bacterial, viral, fungal, protozoal, and helminthic infections (see Ch. 33). 2.Chronic inflammatory diseases (eg, rheumatoid arthritis, polymyalgia etc, see Ch. 32). 3.Malignant disorders. This anaemia is characterised by low serum-iron as well as low total iron binding capacity. A3. Sideroblastic anaemia (defect synthesis of haem) with ring sideroblasts, is genetic or acquired. The genetic type is X-linked and transmitted by the mother. The acquired types are caused by alcohol, drugs, lead, other disorders or the cause is unknown (primary type). Sideroblastic anaemia is characterised normal total iron binding capacity, raised serum-iron and raised serum-ferritin. A4. Macrocytic anaemia with megaloblasts in the bone marrow is due to folate deficiency or to vitamin B12 deficiency. Folate deficiency anaemia is recognised when the folate concentration in red cells low. This deficiency is due to poor intake, malabsorption, antifolate drugs and excess utilization. Since the folate stores of the body are low the anaemia develops rapidly (over months) compared to years for pernicious anaemia. Folate polyglutamates are synthesized in human cells. These compounds are biologically active, as coenzymes in amino acid metabolism and in the DNA synthesis. The synthesis of the biologically active form of folate is dependent of vitamin B12. Lack of folate inhibits the purine-pyrimidine-DNA-synthesis, and without new DNA cell division is seriously reduced. The typical patient appears with glossitis and a megaloblastic anaemia is found. The amount of folate in red cells is below 160 mg ml-1. The normal range is 160-640mg ml-1. Pernicious anaemia is the most common cause of vitamin B12 (cobalamin) deficiency. Pernicious anaemia is characterised by a low serum-[vitamin B12] (below 160 ng l-1). Megaloblastic anaemia with lack of gastric HCl confirms the diagnosis. Pernicious anaemia is caused by atrophy of the gastric mucosa, resulting in insufficient synthesis of intrinsic factor. The stomach cannot secrete intrinsic factor, hydrochloric acid and pepsin. Pernicious anaemia occurs in three forms: 1) most patients have an autoimmune disorder, with plasma antibodies against their own parietal cells; 2) rarely, new-born babies suffer from congenital intrinsic factor deficiency with normal pepsin and acid secretion; and 3) finally as vitamin B12 malabsorption, because of a defect in the intrinsic factor-B12 receptors in the terminal ileum. Vitamin B12 malabsorption in adults is caused by one of two intrinsic factor antibodies. One antibody blocks the binding of intrinsic factor to B12, so the protease-resistant complex is never formed. The other intrinsic factor antibody blocks the binding of the intrinsic factor- B12 complex to the intrinsic factor-B12 receptors of the terminal ileum. The result is vitamin B12 malabsorption. Parietal cell antibodies are present in the plasma of 90% of all patients with pernicious anaemia. The parietal cells of the gastric glands fail to secrete HCl and intrinsic factor. Intrinsic factor is a glycoprotein, which combines with vitamin B12 of the food. This combination normally makes vitamin B12 available for absorption in the ileum. The site of red cell production is the red bone marrow, which is normally one of the most proliferative tissues. The lack of vitamin B12 in the liver and the red bone marrow inhibits the methyl-malonyl Co-A mutase and also spoils the purine-pyrimidine-DNA-synthesis. The inhibition of these and other processes leads to the neurological and the haematological disorders in pernicious anaemia. The neurological features are progressive polyneuropathy with degeneration of the posterior and lateral column of the spinal cord and peripheral nerves (eg, optic atrophy, symmetrical paraesthesia, weakness, dementia and ataxia). Haematological disorders. Lack of vitamin B12 in the bone marrow turns the normal erythroblasts into abnormal megaloblasts. The erythrocyte production is inhibited, and the cells synthesise much more RNA than normal and much less DNA. Besides, the formation of leucocytes and platelets suffer causing leucopenia and thrombocytopenia. Instead of normal erythrocytes, the megaloblasts deliver megalocytes to the circulation. Megalocytes are fragile and only have an average life of 40 days, as compared to 120 days for adult erythrocytes. Cobalamine is the chemical name of vitamin B12. Pernicious anaemia is treated with intramuscular injections of hydroxycobalamin storage, followed by 1 mg every 3 months as long as the patient lives. A5. Macrocytic anaemia without megaloblasts in the bone marrow is a physiological anaemia in pregnancy and in new-born babies. This anaemia is also found in patients with alcohol abuse, hepatic disorders, hypothyroidism, and aplastic anaemia. The concentration of vitamin B12 and folate in the plasma is normal. The relative number of reticulocytes and the MCV is increased. – In some cases there is fat accumulation in the red cell membrane, but the pathogenesis of these conditions is not clarified. A6. Aplastic anaemia refers to a condition of bone marrow failure with only few pluripotent stem cells in the bone marrow. This is due to immune suppression of stem cells by T suppressor cells, or to direct destruction of the stem cells caused by chemicals, drugs, infection or radiation. Pancytopenia, absence of reticulocytes and an aplastic bone marrow is characteristic. A7. Thalassaemia (see Chapter 33). B1. Acute bleeding (loss of red cells). Normochromic normocytic anaemia occurs following an acute bleeding with plasma dilution, before the iron stores are depleted. - Lack of vitamin K can change the development of even a simple tooth bleeding to a serious condition. B2. Haemolytic anaemias (increased destruction of red cells): They are inherited or acquired. Inherited are hereditary spherocytosis or ellipsocytosis, thalassaemia (defect synthesis of globin -see Chapter 33), Sickle syndromes, etc. Acquired haemolytic anaemias are caused by immune destruction of red cells, membrane defects (paroxysmal nocturnal haemoglobinuria, mechanical destruction of cell membranes, haemolysis caused by renal, endocrine or liver disease. Haemolytic anaemia is characterised by osmotic fragility, reticulocytosis, increased serum-bilirubin, and erythroid hyperplasia of the bone marrow. General for anaemia In most cases of anaemia the fall in transport capacity develops slowly, whereby there is time for physiological adaptations to minimise symptoms and signs. A rise in 2,3-DPG improves the release of oxygen to the cells. Unspecific symptoms such as fatigue, headaches and faintness have varying origin and are not always recognised as a disease. Dyspnoea, palpitations, cardiac cramps, and intermittent claudication are also difficult to interpret. The signs of anaemia are tachycardia, systolic murmur over the heart, and cardiac failure. Drumstick fingers with spoon-shaped nails are seen in chronic anaemia with hypoxia such as in chronic iron deficiency. Jaundice suggests the possibility of haemolytic anaemia. The falling red cell count reduces the oxygen delivery but also leads to falling viscosity of the blood. The reduced viscosity can reduce the total peripheral vascular resistance (TPVR) to less than half of the resting value, which is an appropriate event, since it easens the cardiac work and improves the bloodflow. A slight fall in systemic arterial pressure reduces the stimulus of the arterial baroreceptors, and causes a rise in heart rate and cardiac output. The low oxygen capacity of haemoglobin is compensated by an increased coronary bloodflow at rest. The myocardial anoxia results in cardiac failure (Fig. 10-10) with oedema, large liver, and stasis of the neck veins. Severe anaemia increases respiration, metabolic rate, and temperature due to the large cardiopulmonary work. Oedema is an abnormal clinical state characterised by accumulation of interstitial or tissue fluid. Cutaneous oedemas can be diagnosed by the simple test: pitting on pressure. Theoretically, oedemas are caused by three different mechanisms: 1. A hydrostatic pressure gradient, which is too great (so-called high pressure oedema or cardiac oedema at heart failure with increased venous and central venous pressure), 2. A colloid-osmotic pressure gradient, which is too low and caused by too low concentrations of plasma proteins (so-called hunger oedema and renal oedema), and 3. Leakage in the capillary endothelium (so-called permeability oedema with too much protein in the oedema fluid). Burns cause increased capillary permeability for proteins, by infections or by allergy. Cardiac oedema develops in the dependent parts of the human body, where the hydrostatic gradient is greatest (see congestive heart failure, Fig. 10-10). Renal oedema is frequently found in loose tissues, such as the subcutaneous tissue around the eyes (see Chapter 25). Lymphatic oedema is special form of oedema that can be congenital or acquired. A child born with insufficient development of the lymphatic system will suffer from gradual swelling of the affected body part as a result of accumulation of interstitial fluid. Surgical destruction of lymphatic vessels can result in acquired, lymphatic oedema (eg, following mastectomy). Inflammative processes, cancer cells or filarias (elephantiasis) also can obstruct lymphatic vessels, so the limbs swell and become oedematous “elephant limbs.” Thrombosis refers to a condition with formation of multiple thrombi or clots within the vascular system. The cause can be damage of the vessel wall, reduced bloodflow, increased viscosity and hypercoagulability of the blood. Embolism refers to the process through which a thrombus is dislodged from its attachment and travels with the blood until it is lodged in a blood vessel too small to allow its passage. The flowing blood carries emboli from thrombus material in the deep pervic or leg veins to the lungs, where they block the bloodflow as life-threatening pulmonary emboli. Venous thrombosis is is frequently related to peripheral artery disease or to immobilisation. Bed rest or long immobilisation as during long flights can result in deep venous thrombosis, presenting with pain in the calf and ankle oedema. Anticoagulation therapy and elastic support stockings are used to reduce the risk of pulmonary embolism. The bleeding disorders known as haemophilia are relatively seldomly occurring, but vitamin K deficiency must be recognized as a common and serious bleeding disorder, which can give rise to acute bleeding anaemia (B1). Haemophilia A is the most frequent genetic disorder of the intrinsic clotting system, characterised by a low coagulant concentration of antihaemophilic factor (VIII). This disorder is linked to the X-chromosome, and haemophilia affects only males, who transfer the abnormal gene to their daughters, all of whom are carriers. The female carrier of the abnormal gene is usually without symptoms and signs of disease. Haemophilia B (Christmas disease, Factor IX deficiency) is not as common. Most haemophiliacs suffer episodes of spontaneous bleeding. Repetitive joint bleeding (haemarthrosis) leads to crippling arthritis. The activated partial thromboplastin time tests the competency of the slow intrinsic clotting pathway. The contact factors are maximally activated by first mixing citrate plasma with powdered glass. Then partial thromboplastins (V, cephalin, and inosithin) are added. After addition of phospholipid and Ca2+, the time it takes for coagulation to occur is measured. This is a preferential test of the intrinsic clotting pathway, because factor III (tissue thromboplastin from injured cells) is not available to trigger the extrinsic clotting pathway (Fig. 8-6). Normal values are 35-45 s; the time is prolonged in blood from patients with circulating anticoagulants. The time is also prolonged in haemophilia and in other disorders with defective intrinsic pathway factors. Von Willebrands disease. In most forms of Von Willebrands disease the plasma is deficient in both factor VIII and Von Willebrands factor. The disease affects both sexes, which is similar to mild haemophilia. The disorder is inherited as an autosomal dominant trait. The bleeding time tests the capacity of platelets to form plugs. A blood pressure cuff is applied to maintain venous pressure at 5.3 kPa, and a standardised incision is made on the volar surface of the forearm. Bleeding stops when a proper plug of platelets has aggregated. The incision is blotted with filter paper at 30 s intervals. The normal bleeding time is 4.5 min. The bleeding time is prolonged to at least 10 min in Von Willebrands disease. Aneurysms are abnormal dilatations on a vessel typically due to degenerative processes in the wall. Aneurysms on brain or coronary arteries may rupture (leading to sudden death), because of their high lateral pressure (Eq. 8-2). Aortic aneurysms are usually due to arteriosclerosis with large atheromas in the wall. Aneurysms are found as pulsatile dilatations of the abdominal or thoracic aorta (CT scanning or ultrasound examination). Rupture of an aortic aneurysm presents as shock with epigastric pain, and requires immediate surgery. Bleeding inside the wall of the aorta obstructs the lumen (so-called dissecting aortic aneurysm), and also here emergency surgery is required. Left ventricular aneurysm is a complication to ischaemic heart disease often diagnosed by echocardiography (a case is drawn in Fig.10-8). Saccular aneurysms are found on the circle of Willis and its adjacent branches. Pulsations cause pressure on surrounding structures, and spontaneous rupture often causes sudden death. Opening and closure of cardiac valves is studied with echocardiography. This is a versatile non-invasive technique used by cardiologists. When valvular diseases cause the valves to open too little (stenosis) or not close firmly enough (insufficiency), the function of the heart is severely impaired. Valvular disorders are treated in Chapters 10 and 12. · A geometrical argument: The relationship between linear mean velocity (v¯ ) and the bloodflow in one s ( Q° s) is determined by the cross sectional area (A): Eq. 8-1: Q°s = v¯ ×A. · Bernoulli’s equation (see Chapter 13) states that the total driving energy, applied to a continuously flowing, small, ideal fluid volume (dV), which is flowing frictionless and laminar, equals the sum of 3 types of energy the kinetic energy (1/2 r v2 - fluid density (r ) multiplied by the squared velocity) , the potential energy at the height (h) and the gravity (G), and the laterally directed energy (ie, the lateral pressure, P, directed towards the walls). Eq. 8-2: Total energy zero = dV (1/2 r*v2 + h*r *G + P). The lateral pressure is highest, where the velocity is lowest (eg, aneurysm ruptures). The equation of continuity states that the velocity varies inversely with the cross-sectional area of the tube. Consequently, the lateral pressure is highest where the cross sectional area of the tube is largest. Poiseuille´s law: The volume rate (Vdot) is equal to the driving pressure (DP) divided by the resistance: V° = DP/Resistance. For the left ventricle, the bloodflow is actually cardiac output (Q° ), so the equation reads: Eq. 8-3: Q° = DP/TPVR (l min-1). The driving pressure (DP) is the mean arterial pressure (MAP) minus the atrial pressure, and TPVR is the total peripheral vascular resistance. TPVR is directly related to the blood viscosity (h) and to the length (L) of the vascular system, and inversely related to its radius in the 4th power: Eq. 8-3a: TPVR = 8 h L/r4. Doubling the length of the system only doubles the resistance, but halving the radius increases the resistance sixteen-fold. - Poiseuille´s law is an approximation! · Vascular Resistance in parallel organs. In the systemic or peripheral circulation the resistance in the single organs are mainly placed in parallel, and the resistance of all organs (R1 toRn) are related to the total (TPVR) by the following relation: Eq. 8-4: 1/TPVR = 1/R1 + 1/R2 +............1/Rn. · Vascular resistance in portal circulations. There are only a few serially connected elements (portal circulation): Spleen/liver, gut/liver, pancreas/liver and hypothalamus/pituitary. For serial arranged resistance the formula is: Eq. 8-5: Rtotal = R1 + R2 + .........Rn. · The law of Laplace. For a thin-walled organ with two main radii, the relationship between transmural pressure (DP) and tension (T) is determined by the radii: Eq. 8-6: DP = T /(r1 + r2). – See Fig. 8-9. · The Starling equation Starling described the transvascular fluid flow (Jf, volume per min in 100 g of tissue), determined by the combined effect of the Starling forces, in 1896 in the equation: Eq. 8-7: Jf = Capf × [(Pc - Pt) - s(pc - pt)]. Capf is the capillary filtration coefficient (ml of fluid per min per kPa in 100 g of tissue). The Starling forces are the pressure differences in brackets. Pc is the capillary hydrostatic pressure, Pt is the tissue hydrostatic pressure (zero), pc is the capillary colloid osmotic pressure (3.6 kPa or 27 mmHg), pt is the tissue colloid osmotic pressure (0.5 kPa), and s is the capillary protein reflection coefficient. I. Each of the following five statements have True/False options: A. Solutes are exchanged in capillaries and small venules, because of the large surface area and the thin endothelial vessel walls with many pores. B. Oxygen diffuses from the blood to the interstitial fluid mainly across the total surface of the endothelial cell walls. C. Systemic oedema is caused by a small increase in mean arterial pressure. D. The bloodflow through the capillaries is regulated by arteriolar tone. E. Oxygen is a water-soluble gas. II. Each of the following five statements have True/False options: A. Oedema is always caused by a hydrostatic pressure gradient, which is too great. B. Macrocytic anaemia without megaloblasts in the bone marrow is found in pregnancy, in newborn babies, in hepatic disorders, in hypothyroidism and in aplastic anaemia. C. The erythrocyte sedimentation rate is normally only a few mm per first hour, 15-20 with a common cold and 50-100 during pregnancy. D. The reticulocyte count is normally less than 2.5% of the red cell count, but following haemorrhage or haemolysis the relative number of reticulocytes increases reflecting increased erythropoiesis. E. The three-leaflet mitral valve prevents the leakage of blood backward from the left ventricle to the left atrium. III. Each of the following five statements have True/False options: A. The lack of vitamin B12 in the liver and the red bone marrow inhibits the methyl-malonyl Co-A mutase and spoils the purine-pyrimidine-DNA-synthesis. The inhibition of these two processes leads to the neurological and the haematological disorders in pernicious anaemia. B. Mean corpuscular volume expresses the mean volume of each red cell, and mean corpuscular haemoglobin concentration provides the mean concentration in each red cell. C. Pores of 0.4-0.6 mm permeate the venous end of the capillaries. D. Fenestrations are round windows found in the capillaries of organs that transport lots of water (the bowels, glomerular capillaries of the kidneys, pancreas and salivary glands). The protein lattice in the fenestrae is so tight, that it keeps plasma proteins back. E. Newtonian fluids are defined as fluids with a viscosity that is dependent of the shear rate. A grey-haired male with blue eyes, 52 years old, is complaining of precordial pain, Dyspnoea upon stair climbing, and nausea. He is depressed and suffers from frequent coughs. The doctor observes icteric skin and eyes, ataxic walking, dysdiadochokinesis, and positive Babinski. Massive subcutaneous bleeding was found at the left hip. Laboratory tests revealed the following abnormal results: Lack of HCl in the gastric fluid during fasting and following a pentagastrin test. Haematology tests revealed large erythrocytes - many with nuclei. The red cell count was 1.4*1012 per l. The haematocrit was 0.21, and the blood [haemoglobin] was 4 mM. The bleeding time was 90 min and the platelet count was 50*109 per l. The concentration of vitamin B12 in serum was 90 ng per l. The total [bilirubin] in serum was 18 mg per l, and the rise mainly due to non-conjugated bilirubin. A test with radioactive B12 was specific for lack of intrinsic factor production from the patient’s parietal cells. 1. What was the cause of this severe pancytopenia (lack of all blood cell types)? 2. Calculate the oxygen capacity for haemoglobin. 3. Why did the patient develop leucopenia and thrombocytopenia? Was the lack of leucocytes and platelets of any consequences to the patient? 4. Does a severe, chronic anaemia trigger physiologic adaptations? In a healthy 20-year old male, with a mean cardiac output of 7 l per min and a haematocrit of 45%, 20 l of fluid are filtered per day in the capillaries. The concentration of protein in the fluid is 5 g per l. A daily volume of 3 l of fluid passes into the lymphatic vessels and is returned to the blood as lymphatic fluid. The capillaries absorb the rest of the filtered fluid, supposedly together with a small amount of protein (10 g). The total amount of plasma reaching the capillary system every day must be 55% of all the whole blood. Each day has 1440 min, so the plasma flow is: (7*1440*0.55) = 5544 l per day. 1.Calculate the mean protein concentration in the lymphatic fluid. 2.Compare this concentration to that of liver lymph. Try to solve the problems before looking up the answers · Erythrocyte sedimentation rate (ERS) is abnormally increased (above 20 mm) in anaemia, infections, immunology reactions, ischaemia, malignancy or traumata. Normally, the level is only a few mm. · Haemopoiesis is the formation of blood cells. All blood cells are derived from stem cells. Stem cells produce erythroid cells, granulocytes, lymphoid cells, megacaryocytes and monocytes by a number of differentiation steps. Stem cells maintain normal cell populations in a healthy bone marrow controlled by haemopoietic growth factors, and stem cells have the capacity for self-renewal. · The erythropoiesis is controlled by the hormone erythropoietin. Erythropoietin is liberated to the circulating blood as a response to hypoxia of any cause (eg, cardiac-pulmonary-renal disease). · The successive change in affinity during binding of the 4 oxygen molecules to each haemoglobin, explains the sigmoid shape of the oxygen dissociation curve. · When blood is saturated under the normal ambient oxygen partial pressure (20 kPa or 150 mmHg), the oxygen capacity of haemoglobin is 1.34 and not the theoretical maximum 1.39 ml STPD g-1. · In many small vessels bloodflow is non-Newtonian and Poiseuilles law is not applicable. · Bloodflow tends to become turbulent, when the flow velocity is high, the viscosity is low, and the vessels are irregular. · Starling’s paracapillary circulation plays a dominating role in the transport of crystalloids through the capillary wall. · Of the daily capillary filtration, 9/10 is reabsorbed in the venous end of the capillaries, and 1/10 forms the lymph. · Lymphatic fluids from liver and kidney have a protein concentration equal to plasma’s, whereas those from skin and skeletal muscles only contain 2% protein, and brain lymph no protein at all. · Severe anaemia increases respiration, metabolic rate, and temperature due to the large cardiac work. · Aging and arteriosclerosis increase the stiffness of elastic arteries, causing the arterial compliance to fall from 1 to about 0.5 ml of blood per mmHg. · Pernicious anaemia is the most common cause of vitamin B12 (cobalamin) deficiency. This is a disorder with an atrophic gastric mucosa. Parietal cell antibodies are present in the plasma of 90% of all patients with pernicious anaemia. The parietal cells of the gastric glands fail to secrete HCl and intrinsic factor. Pries, A.R., T.W. Secomb, and P. Gaetgens: Design principles of vascular beds. Circ. Res. 77: 1017, 1995. Rostgaard J and K Qvortrup: Electron microscopic demonstrationsof filamentous molecular sieve plugs in capillary fenestrae. Microvascular Research 53: 1-13, 1997.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Click here to introduce your comments or contributions