New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 23 :Hepatic and Pancreatic Function and Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 23
|
 |
|
|
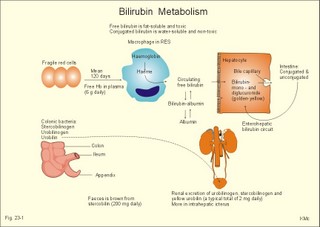
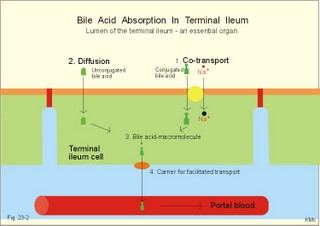
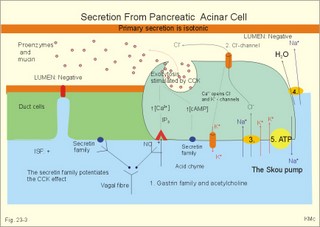
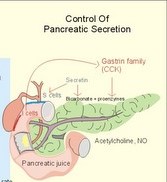
· To define concepts such as ascites, a basic hepatic unit, bile acids, biliary cirrhosis, cirrhosis, hepatitis, icterus, ileus, micelles and pancreatitis. · To describe the normal hepatic and pancreatic function and related clinical tests. · To explain the formation of hepatic bile and pancreatic juice, the entero-hepatic bile acid cycle, the function of the bile bladder, the consequences of insufficient bile secretion, the stimulus-secretion coupling in the acinar cells of the pancreas. To explain the control of bile secretion and of exocrine pancreatic secretion. To explain the pathophysiology of common hepatic and pancreatic disorders. · To use the concepts in problem solving and case histories. · The liver is responsible for the key elements of intermediary metabolism, regulating the metabolism of carbohydrates, lipids and proteins. · Pancreas is the classical mixed gland with both exocrine and endocrine elements. · Ascites refers to abnormal accumulation of transudate in the peritoneal cavity. · Bile acids are detergents synthesized from cholesterol in the liver. Cholic acid and cheno-deoxy-cholic acids are conjugated with glycine and taurine, whereby they become water-soluble. · Biliary cirrhosis is a progressive cholestasis with cirrhosis caused by destruction of bile ducts and bile ductules. Antibodies to mitochondria are found in the blood and the aetiology probably includes immunological phenomena. · Cholelithiasis or gallstone disease is defined as a condition with gallstones within the lumen of the gallbladder, whether symptoms occur or not. · Cholestasis refers to intra- or extra-hepatic obstruction of the bile flow. · Cirrhosis refers to destruction of the normal hepatic lobular structure by fibrous septa, necrotic hepatocytes and regenerative nodules of hepatocytes. · Hepatitis is either infectious or toxic. Virus, bacteria or protozoa cause infectious hepatitis. Liver toxins, haemolytic toxins, metabolic toxins and drugs cause toxic hepatitis. · Icterus refers to yellow coloration of skin, blood plasma, mucous membranes and tissues. The threshold for visible jaundice (icterus) is a [bilirubin] in blood plasma above 18 mg l-1 or 30 mM in most people. · Ileus means intestinal obstruction either due to blockage of the intestinal lumen or to damage or death of smooth muscles that results in paralysis (paralytic or adynamic ileus). The intestine proximal to the lesion is dilatated by fluid and chyme. · The liver lobule is the basic hepatic unit consisting of the hepatic triad: Centrally located is the central vein with columns of hepatic cells arranged in radials. Branches of the hepatic artery and the portal vein are located on the periphery of the lobule. Blood from these vessels perfuse the sinusoids between the hepatocytes. · Micelles are molecular vehicles, consisting of amphipathic bile acids (bile acid micelles) and often aggregates of lipids (mixed micelles). Amphipathic molecules have a hydrophilic and a hydrophobic surface. · Pancreatitis is an inflammatory disease with interstitial oedema in mild cases, and necrosis of the acinar cells of the pancreas in severe cases. The inflammation is not seriously affecting the pancreatic islets. This paragraph deals with: 1. Bile, 2. bile acid production, 3. bile pigment production, and 4. pancreatic exocrine secretion. The basic hepatic unit is the liver lobule. The liver cells (hepatocytes) are arranged into walls of cells, which are separated by highly porous capillaries (Fig. 8-11) called venous sinusoids. The portal vein brings blood from the intestine, and the hepatic artery brings arterialised blood from the heart. The venous sinusoids are lined with fenestrated endothelial cells and with specialised, reticuloendothelial Kupffer-cells. The large demand of O2 and nutrients is satisfied from this pool of mixed blood by the hepatocytes; then the blood drains into the central veins and leaves the liver through the hepatic vein. The hepatocytes form bile and secrete it into bile canaliculi, which converge to form a ductal system, where bile flows in the opposite direction of the blood. Hereby, cleared blood passes "new" bile. The many bile ducts converge to form the hepatic duct. Secretin stimulates the secretion of bicarbonate from the bile ductal system. Bile has a golden colour and is nearly isotonic with blood plasma. The bile contains NaCl and bicarbonate in concentrations similar to those of plasma, but the bile contains more Ca2+ (bound to bile acids) than plasma. We normally produce 0.5-1 litre of hepatic bile per day with bile salts, lecithin, cholesterol and 1.5 g of bile pigments. The normal gall bladder can concentrate the hepatic bile by a factor up to 5. The bile salts (Cholic acid and Deoxy-cholic acid) are made from cholesterol, which is also abundant in bile. The formation of mixed micelles (special fatty aggregates) containing cholesterol, phospholipid, and bile salts, provide concentrations of both phospholipid and cholesterol far exceeding their normal solubility in water. Cholecystokinin (CCK) is released by the duodenal mucosa in response to contact with fat and essential amino acids. CCK reaches the gallbladder wall via the blood, and it causes contractions of the gall bladder and relaxation of the sphincter of Oddi. Gastrin has a small CCK-effect, and VIP/acetylcholine inhibits gallbladder contractions. Red blood cells have a life span of 120 days and are continuously being degraded, and the haeme released is taken up from the blood by the hepatocytes to produce bilirubin. Bilirubin is conjugated to glucuronic acid by a transferase in the liver cell to form the golden yellow bilirubin mono- and di-glucuronide. These conjugates are much more water-soluble than bilirubin, and are thus easily excreted by the bile capillaries (Fig 23-1). The liver contains a large store of vitamin B12. Only 0.1% of the store is lost daily in the bile, because most of its content is reabsorbed in the terminal ileum. Even if absorption totally ceases the hepatic vitamin B12 store lasts for 5-6 years. In the absence of vitamin B12 the maturation of erythrocytes is retarded, and pernicious anaemia develops (Chapter 8). Bile acids are detergents synthesized from cholesterol in the liver. Cholic acid and cheno-deoxy-cholic acid are conjugated with glycine and taurine, whereby they become water-soluble. Bile acids have lipophilic and lipophobic terminals, which increase the lipid solubility of the intestinal chyme by micelle formation. Phospholipids and cholesterol expand simple micelles into effective mixed micelles. In the terminal ileum intestinal bacteria change the two major bile acids into deoxy-cholic acid and litho-cholic acid. The bile acid production is reduced without the return of bile acids from the terminal ileum and steatorrhoea develops. Cholate and desoxycholate are fat-soluble agents. They have fat-soluble hydrocarbon rings that enable them to mix with fats and several charged groups that enable them to mix with water. Large fat droplets in the duodenal chyme become dispersed, forming smaller fat particles - a process called emulsification. The bile salts contribute to emulsification. These smaller fat particles are efficiently digested by the water-soluble pancreatic lipases, forming glycerides and fatty acids in the micelles (Fig. 22-13). The micelle contents are readily absorbed by the enterocytes. The co-lipase helps the lipase to eliminate the inhibitory bile salts from the surface, so that the lipase is fixed to the lipids. The pancreatic lipase cleaves the ester linkage of tri-acyl-glycerol at the 1- and 3-position, releasing two fatty acids and 2-monoglyceride (2 MG), or occasionally a free glycerol molecule (Fig. 22-13). Free glycerol is readily absorbed. A protein - fatty acid binding protein (FABP; 12 kDa) - is present in the cytosol of the enterocytes. FABP binds fatty acids in order to re-esterify the fatty acids and to protect the cell from adverse effects of cytotoxic fatty acids. Once the fatty acids are formed, the fatty acids and 2-monoglyceride participate in the emulsification process, but the fatty acids still require bile salts for complete water solubility. Micelles are passed through the aqueous bowel lumen to reach the absorbing mucosa (Fig. 22-13). A large concentration of bile salts helps the lipid laden micelles to get access to the absorbing surface. Then lipids diffuse easily out of the lipophilic micellar core and into the lipid layer of the apical membrane of the mucosal cell. Lipids do not adequately form micelles, if there is no bile present. 3. Bile pigment production and excretion Mature red cells are continuously being degraded in the macrophages of the liver and in the reticulo-endothelial system (RES) of the spleen and bone marrow. The haeme is converted to biliverdin, which is reduced to produce bilirubin (Fig. 23-1). About 10-15% of the bilirubin arises from the breakdown of immature red cells and cytochrome. Bilirubin released to the blood from these tissue macrophages is bound to albumin during its transport, so the concentration of neurotoxic, free bilirubin is normally low. Bilirubin is taken up at the hepatocyte cell membranes after dissociation from the albumin. Within the hepatocyte bilirubin is transferred to the endoplasmic reticulum, which contains a transferase that conjugates bilirubin with glucuronic acid. Conjugated bilirubin (bilirubin di- and mono-glucuronide) is water-soluble, so it diffuses easily through the cytoplasm and is actively secreted into the bile canaliculi. From here conjugated bilirubin is excreted into the intestine with the bile. Bacterial enzymes in the terminal ileum and the colon hydrolyse the large molecule. The free bilirubin is then reduced to urobilinogen. Most of the urobilinogen is excreted in the faeces; the remainder is absorbed in the terminal ileum, returned to the liver via enterohepatic blood, and again excreted as bile into the intestine (the enterohepatic bile pigment circuit). A small amount of the urobilinogen is excreted in the normal urine. Fig. 23-1: Normal bilirubin metabolism. The free bilirubin is fat-soluble and toxic. The conjugated bilirubin is water-soluble bilirubin-glucuronide and non toxic. Part of the bilirubin is broken down to colourless substances, hepatocytes produce urobilinogen, and colonic bacteria stercobilinogen. Both substances can be oxidised to yellow urinary urobilin and brown faecal stercobilin (Fig. 23-1). The renal excretion of urobilin and stercobilinogen is increased in cases with intrahepatic icterus (ie, hepatitis and other damages of hepatocytes). The terminal ileum is essential for life. 1. The terminal ileum of humans absorbs conjugated bile salts efficiently by an active Na+ -dependent co-transporter that is similar to the glucose/Na+ - and amino acid/Na+ -co-transporter in the duodenum-jejunum (Fig. 23-2). Fig. 23-2: Absorption of bile acids by the terminal ileum. 2. Bile acids also cross the brush border by diffusion in unconjugated form. 3. In the cytosol the bile acids are probably bound to macromolecules, and they traverse the basolateral membrane by 4. facilitated transport and diffusion into the portal blood. The absorbed bile salts reach the liver, where they are conjugated and reprocessed, and the hepatocytes clear the portal blood from bile acids in a single passage. The reabsorbed bile acids are essential stimuli for the liberation of bile with new (15%) and reprocessed (85%) bile acids, but when entering the liver they inhibit the synthesis of new bile acids. The total bile acid pool in the body is about 3 g, and this pool can be recycled up to 12 times per day. By contrast, the intestine reabsorbs only a small part of the bile pigments - the enterohepatic bile pigment circuit (Fig. 23-1). 4. Pancreatic exocrine secretion PAN KREAS is Greek and means all meat. Pancreas is the classical mixed gland with both endocrine and exocrine elements. The exocrine pancreas is an abdominal “salivary gland.” The endocrine pancreas is described in Chapter 29. Secretions from the zymogen containing acinar cells collect in the acinar duct and travel through a network of converging ducts to the main pancreatic duct, which run into the common bile duct entering the duodenum at the duodenal papilla (Vateri), where the sphincter of Oddi is located. The exocrine glandular tissue consists of acinar cells producing a primary secretion, with an ionic composition similar to that of plasma, and duct cells forming the secondary secretion by modification of the primary secretion. The organic components, secreted by acinar cells, are the major enzymes necessary for digestion of dietary nutrients. The acinar cells also secrete mucus and ions. Fig. 23-3: Secretion of enzymes from pancreatic acinar cells. ISF means interstitial fluid.
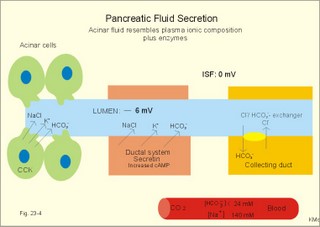
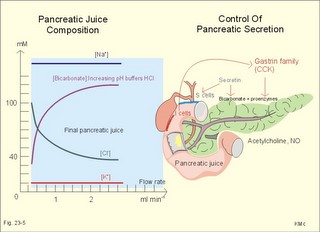
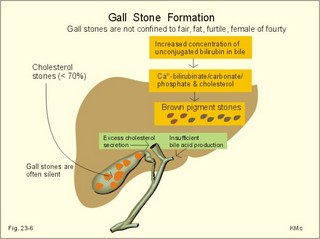
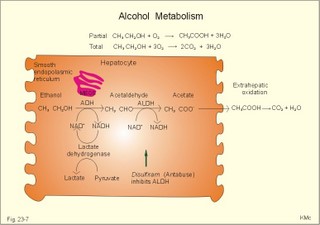
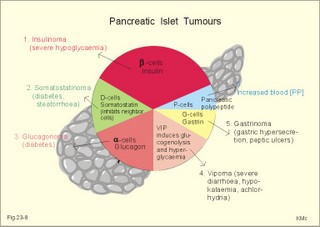
As the primary juice leaves the acini and proceeds down the pancreatic ducts, it is supplied isotonically with water and electrolytes (mainly bicarbonate salts) from the duct cells (Fig. 23-4). Fig. 23-4: Secretion from pancreatic duct cells. Upon stimulation of the duodenal mucosa with acid chyme, secretin is secreted to the blood and transported to the pancreatic duct cells. This induces an important rise in cellular [cAMP]. The rise in [cAMP] activates luminal Cl-- and basolateral K+-channels, so these ions leave the cell in a balanced relationship. This triggers a luminal Cl-/HCO3—co-exchanger through which the cell eliminates the bicarbonate produced by carbon dioxide from the blood. Cellular carboanhydrase is essential for the bicarbonate production. A certain luminal [Cl-] is necessary for recycling. The net result is a secretion of bicarbonate. This net secretion of bicarbonate induces a (lumen negative) transepithelial potential difference (-6 mV), which constitutes the driving force for the paracellular transport of Na+ and K+. The net secretion of salt drags water trans-epithelially in isosmotic proportion. The fall in cellular pH upon secretion of bicarbonate activates a basolateral Na+/H+-coexchanger, whereby the cells eliminate H+ to the blood. The secretory energy is from the basolateral Na+-K+-pump. The whole system is analogous to the formation of saliva. The pancreas (weight 100 g) of adult humans is capable of elaborating approximately 1.5 l of pancreatic juice daily, and its pH increases with increasing secretion rate. The maximal secretion rate is one ml/g of tissue each hour (ie, 60 times less than that of the salivary glands).The pancreatic juice is a clear fluid, isosmolar with plasma. The basic reaction is due to bicarbonate, and the [bicarbonate] can approach the [H+] in gastric juice (150 mM). Fig. 23-5: Concentrations of ions (mM) in pancreatic juice as a function of the secretory flow rate (left). – The control of pancreatic secretion is shown to the right. With increasing secretion rate, the [bicarbonate] in the final pancreatic juice increases at the expense of [Cl-], whereas the [Na+] and [K+] remain relatively constant (Fig. 23-5). Pancreatic juice (pH 8) thus buffers the extremely acid gastric juice and protects the duodenal mucosa against erosion. Buffering of gastric juice also optimises the activity of pancreatic digestive enzymes in the duodenum. The pancreatic secretion is regulated by two intestinal hormone families (Fig. 23-5, right): The secretin- (secretin and VIP) and the gastrin-family (gastrin and CCK), as well as by the autonomic nervous system. Signals in cholinergic, vagal fibres stimulate both pancreatic secretions via acetylcholine-receptors (Fig. 23-3), whereas noradrenergic, sympathetic stimuli inhibit secretion via a-receptors. The secretion is also stimulated by signals in peptidergic nerve fibres. The free radical gas nitric oxide (NO) stimulates the exocrine pancreatic secretion, and simultaneously inhibits the non-adrenergic, non-cholinergic intestinal activity (Fig. 22-6). Related to the meal there are three phases of pancreatic secretion (cephalic, gastric and intestinal). 1. The cephalic phase is elicited before food reaches the stomach. Olfactory signals (via the limbic system) as well as visual and tactile signals (via the thalamic relay station) are processed in the brain, and vagal signals reach the antral mucosa. Here gastrin is released from G-cells. Gastrin induces the secretion of a low volume of pancreatic juice with a high enzyme content. 2. The gastric phase is elicited by the presence of food in the stomach. Gastric distension and peptides reaching the antral mucosa trigger the release of more gastrin from the G-cells. Hereby, the secretion of a small volume of pancreatic juice rich in enzymes is continued. 3. The intestinal phase is elicited by duodenal and jejunal mechanisms. When chyme enters the duodenum both secretin and CCK is released for different reasons. Secretin is secreted by S-cells in the mucosa of the upper small intestine, when acid chyme (pH below 4.5) arrives to the first part of the duodenum. This is an appropriate arrangement, because secretin stimulates both the secretion of bicarbonate and water by pancreatic duct cells (Fig. 23-4), and of bicarbonate-rich bile by small biliary ductules. Secretin inhibits gastric secretion. Secretin inhibits both the gastrin release by the antral G-cells, and the gastrin effect on the parietal cells. CCK from the duodenal I-cells stimulates gallbladder contraction as its name implies, and stimulates pancreatic acinar secretion of an enzyme-rich fluid (Fig. 23-3). The most important stimulus for CCK liberation is when an acid chyme with amino acids, peptides and long chain fatty acids reach the duodenal mucosa. This is essential. CCK contracts the gallbladder and stimulates the pancreatic secretion of an enzyme rich juice. Bile is ejected into the duodenum, where fat is emulgated to ease absorption. CCK also acts as an enterogastrone - an intestinal hormone that inhibits gastric activity and emptying. This leaves more time for the bile to emulgate fat and for the digestible enzymes to work. Pancreatic a-amylase does not pose any danger to pancreatic tissue. Pancreatic a-amylase - like the salivary a-amylase - cleaves the large dietary carbohydrate molecules at the internal 1,4-glycosidic bonds, but cannot hydrolyse terminal 1,4-bonds or 1,6-bonds. The end-products are oligo- and di-saccharides like maltose (two glucose), maltriose (three glucose) and branched oligosaccharides known as a-limit dextrins. Other enzymes such as maltase and lactase secreted by the intestinal mucosa digest these end-products into monosaccharides (glucose, fructose and galactose). The carbohydrate absorption is shown in Fig. 22-11. The protein digestion is continued in the duodenum and jejunum, where the protein breakdown products are attacked by the proteolytic enzymes of the pancreas (trypsin, trypsinogen, chymo-trypsinogen, pro-carboxy-peptidase, and pro-elastase). The pancreatic proteases are secreted as inactive proenzymes, and they are crucially important. The proenzymes are normally not activated before they arrive in the intestinal lumen. Trypsin catalyses its own activation (autocatalysis), and also activates chymotrypsinogen and the pro-carboxypeptidases in the trypsin cascade. Duodenal enterokinase cleaves trypsinogen to trypsin, and hereby activates the trypsin cascade. When the chyme is pushed into the duodenum, the pancreatic juice neutralises the chyme and the pepsin activity is stopped. The peptides and amino acids are absorbed as shown in Fig. 22-12. Normally, trypsin inhibitor inhibits the trypsin cascade from the pancreas, but cases of acute pancreatitis cannot inhibit the trypsin cascade, so autodigestion occurs. Enzymes for the breakdown of fats are pancreatic lipase, phospholipase A, and lecithinase. Pancreatic lipase and co-lipase cleave triglycerides into free glycerol and fatty acids or to mono-glycerides (MG) and fatty acids. Free glycerol is readily absorbed. The lipolytic activity requires the emulsifying action of bile salts in order to solubilize triglycerides in water. Once liberated fatty acids and mono-glycerides participate into bile salt micelle formation. Micelles pass by diffusion through the unstirred water layer of the intestinal lumen to reach the absorbing mucosa (Fig. 22-13). This paragraph deals with 1.Jaundice, 2.Gallstones, and 3. Hepatitis, 4. Liver cirrhosis, 5. liver cancer, 6. Pancreatitis, 7. Cystic fibrosis, 8. Carcinoma of the pancreas, 9. Endocrine pancreatic tumours. Bilirubin has a molecular weight of 588 g per mol. The normal [bilirubin] in blood plasma is up to 17 mg l-1 or 29 mmol l-1 (mM). The threshold for visible jaundice (icterus) is a [bilirubin] in blood plasma above 18 mg l-1 or 30 mM in most people. Three types of icterus can be distinguished: 1.a. Prehepatic or haemolytic icterus is caused by excessive destruction of mature or immature red cells. Haemolytic anaemia causes haemolytic jaundice. Increased destruction of red cells (haemolysis) increases the bilirubin production (normally 35 * 6 = 210 mg daily) to the extent that the hepatocytes cannot conjugate the bilirubin as rapidly as it is formed (the key hole enzyme is glucuronyl transferase). The neurotoxic free bilirubin in blood plasma rises much above normal, and large quantities of urobilinogen is excreted in the urine. 1.b. Intrahepatic icterusis caused by poor hepatocyte function. Damages of the hepatocytes by infections, tumours, or toxic agents impair the uptake, transport and conjugation of bilirubin. Absence of glucuronyl transferase or inhibition of the enzyme by steroids block conjugation of bilirubin. 1.c. Posthepatic icterus is caused by cholestasis due to gallstones or pancreatic tumours. Gallstones or tumour masses obstruct the bile ducts, which is causing extrahepatic cholestasis with impaired excretion of conjugated bilirubin to the intestine. Hereby, conjugated bilirubin reflux to the blood. Most of the bilirubin in plasma is therefore conjugated and some of it strongly bound to plasma albumin. Hepatic Failure results from destruction of liver cells or impairment of hepatocyte function. Liver failure causes severe jaundice, hepatic encephalopathy, the hepatorenal syndrome, pulmonary veno-arterial shunts, and low coagulability of the blood. Cholelithiasis or gallstone disease is defined as a condition with gallstones within the lumen of the gallbladder, wether symptoms occur or not. More than 70% are cholesterol stones; the remainder is a so-called brown pigment gallstone composed of Ca2+ salts of bilirubin, carbonate, cholesterol and phosphate (Fig.23-6). Fig. 23-6: The two common types of gallstones: Cholesterol stones and brown pigment stones. Approximately half of the pigment stones are radiopaque. The incidence of cholesterol stones among females is three times higher than among males. The cause is both genetic and environmental. Oestrogens stimulate hepatic secretion of cholesterol and reduce the formation of bile acids. Diets high in cholesterol increase the incidence of gallstones. Bile can be supersaturated with cholesterol. When this happens, crystals can precipitate out of bile. Cholesterol crystals and Ca2+ can aggregate and develop into gallstones in the common bile duct. Mixed gallstones and gallstones made of bile pigment and other bile substances are also found. Excessive removal of water in the gallbladder can be pathogenic. Enlarged gallstones can obstruct the common bile duct thus causing bile with bilirubin to flow back into the liver and leak into the blood plasma (jaundice or icterus). Most gallstones are asymptomatic, but those occluding the biliary tract cause a severe pain called biliary colic. Hepatitis is either infectious or toxic. Virus, bacteria, or protozoa cause infectious hepatitis. Virus molecules cause most of all global hepatitis (see Chapter 33). Bacterial and rickettsial hepatitis is caused by leptospirosis interrogans icterohaemorrhagia (Weil-hepatitis), rickettsia (typhus-hepatitis), streptococci, pneumococci etc - sometimes by ascending infection after biliary tract disorders. Protozoan hepatitis is caused by the species of plasmodium in malaria-hepatitis, trypanosoma in trypanosomiasis, and toxoplasma gondii in toxoplasmosis-hepatitis. The clinical course is often like infectious mononucleosis. Toxic hepatitis is caused by liver toxins, haemolytic toxins, drugs (isoniazid, methyl-dopa, nitrofurantoin, oxyphenisatin) and metabolic toxins (ileus, ulcerative colitis, pregnancy hyperemesis, thyrotoxicosis). Cirrhosis is the end result after necrosis of the hepatocytes, with destruction of the normal lobular structure by fibrous septa and regenerative nodules of hepatocytes. The clinical picture includes liver failure and signs of portal hypertension such as oesophageal varicose veins and ascites. The terminal stage is hepatic coma. Two pathological types are considered: a. Micronodular cirrhosis is characterized by nodules less than 3 mm in diameter. This disorder was previously termed Laennec’s cirrhosis (after a French pathologist). The cause is alcohol abuse (alcoholic cirrhosis) or biliary tract disease (biliary cirrhosis). b. Macronodular cirrhosis is characterized by larger nodules sometimes including normal lobules. The cause is acute and chronic hepatic infection (hepatitis B virus, hepatitis C virus, hepatitis D virus) often in carriers. This type of cirrhosis follows years of alcohol abuse with fatty liver and alcoholic hepatitis. Fig. 23-7: Alcohol metabolism and fat accumulation. Ethanol is metabolised by the liver hepatocytes to acetaldehyde and acetate. At rest 5% of the molecules are excreted unchanged in the urine, sweat and expired air. The major route of ethanol oxidation is via alcohol-dehydrogenase an NAD-dependent enzyme (Fig. 23-7). A minor pathway is microsomal ethanol oxidising system (MEOS) in the smooth endoplasmic reticulum using NADP as a cofactor. Both enzyme systems are easily saturated, so a fixed quantity is metabolised per time unit (7 g per hour as an average). Both pathways result in an increased NADH/NAD ratio, whereby the fatty acid synthesis is increased. This leads to fat accumulation with fatty liver and alcoholic cirrhosis. The altered redox-potential and the accumulation of acetaldehyde, causes centrilobular necrosis of the micronodular type. The primary type is a progressive cholestasis with cirrhosis caused by destruction of bile ducts and bile ductules. Antibodies to mitochondria are found in the blood and the aetiology probably includes immunological phenomena. There is a high total cholesterol (especially HDL), pruritus, xanthomas, osteoporosis, steatorrhoea, and portal hypertension. The condition is associated with many autoimmune diseases such as rheumatoid arthritis, scleroderma, renal tubular acidosis, and membranous glomerulonephritis (Chapter 32). The secondary type of biliary cirrhosis result from maintained extrahepatic cholestasis. Cirrhosis related to hepatitis The most frequent cause is viral hepatitis in particular infection with hepatitis B virus (also hepatitis D and C virus).Viral markers are examined in the blood such as HBsAg or its antibodies, or IgM anti-HCV (Chapter 33). Seemingly healthy carriers of HBV and HCV are at risk of developing hepatocellular carcinoma. The a-foetoprotein is raised in the blood plasma. Other risk factors for hepatocellular carcinoma are alcoholic damage, haemochromatosis, aflatoxin from peanuts, androgens, and oestrogens. Metastases from breast cancer, bronchial cancer and gut cancer to the liver are much more frequent than primary hepatic tumours. Pancreatitis is an inflammatory disease with interstitial oedema in mild cases, and necrosis of the acinar cells of the pancreas in severe cases. The incidence of pancreatitis in alcoholics is high, whereas infection as such does not account for many cases. Both the first acute case of pancreatitis and the chronic cases are linked to alcohol abuse. a. Acute pancreatitis is characterised by foci of necrotic fat cells besides the acinar cell necrosis and the infiltration of polymorpho-nuclear leucocytes. The injured acinar cells release digestive enzymes (amylase, lipase, and protease) into the blood stream and into the peritoneal fluid causing ascites. Severe epigastric pain is referred to the back between the shoulder blades. The patient is critically ill and develops a shock condition, which may end in terminal renal failure. The diagnosis relies on a 500% rise in serum amylase concentration together with the demonstration of amylase in the abundant peritoneal fluid (ascites). Premature activation of the pancreatic digestive enzymes causes the pancreas to digest itself. The essential enzymes are trypsin, phospholipase A and elastase. The normal balance between trypsin and trypsin inhibitor is destroyed. Protease inhibitors such as a1-antitrypsin, C1- esterase and trypsin inhibitor bind to the proteases and reduce their enzyme activity until the inhibitors are digested by the enzymes. Phospholipase A destruct cell membranes and converts lecithin to the cytotoxic lysolecithin. Vessel walls are broken down and haemorrhage can be fatal. Capillary destruction causes anoxia and necrosis. Alcohol (and acid chyme) stimulates the secretion of secretin from the duodenum and thus the secretion of an enzyme-rich pancreatic fluid. Simultaneously, alcohol closes the sphincter of Oddi, and pancreatic secretions from the obstructed duct are filtered into periductal tissues. The treatment is symptomatic with intravenous nutrition and analgesia. Adequate therapy of shock and respiratory insufficiency must be instituted (transfusions, endotracheal intubation and oxygen if necessary). b. Chronic pancreatitis is a progressive destruction of pancreatic acini, followed by irreversible fibrosis and possibly calcification of the pancreatic tissues. Calcifying pancreatitis is strongly linked to alcohol abuse. The patient has episodes of epigastric pain, and the anorexia results in severe malnutrition. Most patients with calcifying pancreatitis develop steatorrhoea (a copious fatty faeces) and diabetes. Steatorrhoea is treated with low-fat diet and the enzyme, pancreatin, to each meal. The diabetic condition is frequently characterized by a simultaneous lack of pancreatic insulin and glucagon, whereby the daily, external insulin requirement is increased. Alcoholics with pancreatitis must stop drinking alcohol. Pancreatic failure is caused by pancreatitis (inflammation not seriously affecting the Islets), blockage of the pancreatic duct, pancreatic carcinomas and surgical removal of the pancreatic head. Loss of pancreatic juice means lack of pancreatic lipase, pancreatic amylase, trypsin, chymotrypsin, carboxy-poly-peptidase, and elastase. Lack of these enzymes means that half of the fat entering the small intestine pass unabsorbed to the faeces, and one third of the starches and proteins. Steatorrhoea is found. The major metabolic disorder caused by loss of pancreatic endocrine secretion is diabetes mellitus. Removal of pancreas is compatible with survival as long as both the exocrine and endocrine vital substances are supplied artificially. 7. Pancreatic cystic fibrosis (mucoviscidosis) This is a recessive genetic defect with dysfunction of exocrine glands (see Chapter 31). This is almost exclusively adenocarcinoma originating from the duct cells of the pancreatic head. Its occurrence is related to alcohol abuse. The diagnosis is made by CT or by ultrasound technique. Surgical removal of pancreatic adenocarcinoma is frequently unsuccessful. Fig. 23-8: Endocrine pancreatic tumours from the pancreatic islet cells. 9. Endocrine pancreatic tumours 1. Insulinomas are islet cell tumours of b-cells, which release sufficient insulin into the blood to induce serious hypoglycaemia. The patient must eat extensively in order to survive, so obesity is almost unavoidable. 2. Somatostatinomas are islet cell tumours of D- or d-cells that secrete somatostatin to the blood. Somatostatin causes diabetes, steatorrhoea, gallstones and hypo-chlorhydria. 3. Glucagonomas are islet cell tumours of a-cells that release large amounts of glucagon into the blood stream. This causes diabetes, anaemia and a typical erythematosus rash. 4. Vipomas produce large quantities of VIP, and the diagnosis is made by a high plasma VIP. VIP increases the intestinal secretion and causes watery diarrhoea with loss of K+ and H+. Localisation and removal of the vipoma is efficient. 5. Gastrinomas (Zollinger-Ellisons syndrome) consist of G-cells in the pancreatic islets. The G-cells produce large amounts of gastrin causing extensive gastric HCl secretion and peptic ulcers. The serum gastrin is high. The patient has diarrhoea, because of the high H+ -concentration in the intestinal lumen. Omeprazole inhibits the proton pump, whereby the gastric HCl secretion is blocked. 6. PP-producing tumours. The concentration of pancreatic polypeptide (PP) in plasma is increased. PP is released by most pancreatic islet cell tumours, and its plasma concentration is always measured in screenings of islet cell tumours. PP stimulates the gastrointestinal enzyme secretion and inhibits smooth muscle contraction. Localisation and ablation of the tumour is the ideal therapy, but steroids and octreotide are of help. · For calculation of hepatic bloodflow see the Fick Principle in Chapter 10. I. Each of the following five statements have False/True options: A. With increasing rate of pancreatic secretion its [Cl-] will increase. B. Carboanhydrase is an important enzyme for the secretion of pancreatic bicarbonate. C. Duodenal acidification stimulates the pancreatic secretion. D. Duodenal chyme with a pH of 7 inhibits pancreatic bicarbonate secretion. E. Duodenal enterokinase cleaves trypsinogen to trypsin. II. Each of the following five statements have False/True options: A. An elevated concentration of cAMP in the intestinal mucosal cells inhibits Na+ absorption. B. The intestinal Na+ absorption is parallel to the Cl- absorption. C. Increased intracellular [Ca2+] increases intestinal Na+ absorption. D. The basolateral Na+-K+-pump (ATPase) maintains an essential electrochemical gradient with a high intracellular [Na+]. E. The intestinal Na+ absorption is secondary to the water transport across the mucosal cells. III. Each of the following five statements have True/False options: A: Bile acids are essential for solubilizing cholesterol and phospholipids by formation of micelle aggregates. B: Bilirubin binds to cytoplasmic proteins within the hepatocyte. C: The primary bile acids are deconjugated and dehydroxylated to form the secondary bile acids. D: Intrinsic factor-cobalamin complexes are inactivated by pancreatic proteases. E: Cholate and desoxycholate have water-soluble hydrocarbon rings. IV. Each of the following five statements have False/True options: A: Hexoses and amino acids require Na+ for active transport into the enterocyte. B: A person with lactase deficiency cannot digest lactose, so undigested lactose from a milky diet would enter the colon. C: The Na+-K+-pump is essential for intestinal Na+ absorption. D: All lipase proteins are lipid soluble. E: Cytosolic peptidases from the enterocytes and brush border peptidases cannot cleave small peptides into single amino acids. A male patient with coecal cancer still maintains his colon function. Approximately 1.5 l of intestinal fluid passes the ileocoecal valve in 24 hours, and only 150 ml is found in the daily faeces. The intestinal fluid has a [Na+] and [K+] of 120 and 4 mM, respectively. The 75% water in the faecal volume of 150 ml contains 20 mM Na+ and 5 mM of K+. 1. Calculate the water absorption in the colon and rectum. 2. Calculate the net Na+-and K+-absorption in the colon. 3. Calculate the loss of Na+ and K+ with faeces. 4. Removal of the coecum and the ascending colon with the tumour necessitates an ileostomy. Calculate the loss of Na+ and K+ with an unchanged fluid flux through the terminal ileum. 5. Are dietary measures important for the ileostomy patient? A female, age 42 years, is admitted to hospital due to fatigue. She describes a serious gastrointestinal infection for which she was cured some years ago. Suspicion of vitamin B12 deficiency reveals a seriously low vitamin B12 concentration and plasma antibodies against her parietal cells in the gastric mucosa. Assume that the absorption of vitamin B12 totally ceased at the time where her parietal cells were destroyed by autoimmune disease. Assume further that she had a normal liver store of 5 mg vitamin B12, and that she has lost 1 permille daily of the hepatic store in the bile. 1. Calculate the half-time period necessary to reduce the hepatic vitamin B12 store by 50%. 2. Calculate the number of years it takes to empty the hepatic vitamin B12 store down to 0.5 mg (manifest pernicious anaemia). An alcoholic male with hepatic insufficiency is brought to the intensive care unit of a hospital in hepatic coma. Normally, ammonia is formed in the gastrointestinal tract as a product of protein digestion and bacterial action. The liver usually removes a major portion by converting ammonia into urea. Hereby, the toxic ammonia is eliminated. The impaired liver function of this patient has lead to development of collateral venous shunts with oesophageal varicosities. Large quantities of blood from the gut, with a high [NH4+], are transported directly into the systemic veins and the brain of the patient. His blood [NH4+] is drastically increased, and his blood [glucose] is 2 mM (hypoglycaemia). 1. What is hepatic coma? What is causing the unconsciousness? 2. Explain his condition in terms of abnormal glucose metabolism. Try to solve the problems before looking up the answers. · The liver controls the intermediary metabolism of carbohydrates, lipids, and proteins. The liver produces important plasma proteins including coagulation factors, angiotensinogen, trypsin-inhibitor etc. · The liver is a vital glucose exchanger for the hypothalamic glucostat. · The hepatocytes secrete bile (CCK-stimulated) into the bile capillaries and the bile finally enters the small intestine. Bile acids facilitate fat digestion and absorption. Bile acids emulsify lipids, so they are easily accessible for the action of lipid-digesting enzymes. · Bile acids form micelles, which can diffuse to the brush-border membrane for absorption. · Bile acids and vitamin B12 are absorbed in the terminal ileum and returned to the liver in the portal vein. · Hepatocytes clear the blood for bile acids and they are recycled several times daily (enterohepatic recycling). · The bile duct cells and the pancreatic duct cells secrete a bicarbonate-rich fluid (secretin-stimulated). · The liver has an important excretory function, because the hepatocytes excrete bile pigments, and deactivate hormones, toxins and drugs by hydroxylation, proteolysis and hydrogenation. Lipophilic drugs are converted into water-soluble drugs that are easily excreted in bile or urine. · The hepatic reticuloendothelial system normally stimulates repair of tissue damages through influence on T- and B-lymphocytes. Intrahepatic disease impairs the normal immune response to infection elsewhere. · The reticuloendothelial system of the liver eliminates microbes and other antigens transferred to the liver with the blood from the intestines. Antigens are phagocytized by macrophages attached to the endothelium (Kupffer-cells). The macrophages produce collagenase, hydrolases, and interleukins and tumour necrosis factor that degrade the antigens without formation of antibodies. · The normal hepatic store of vitamin B12 is sufficient for 3-6 years, and there is also an important store of other vitamins (A, D, and K). Coagulation factors are stored as well as iron in ferritin. · Approximately half of the total lymph produced in the body is liver lymph, although the liver is only 1.5 kg of the total body weight. Chylomicrons filled with lipids reach the blood via the liver lymph and liver. · The primary oxidation of alcohol (ethanol) occurs in the hepatocytes. Since the alcohol-dehydrogenase activity has a maximum capacity, the elimination rate is constant (0.0025 permille per min). · A pressure rise in the hepatic veins from zero (normally) to 5 mmHg (0.7 kPa) result in hepatic stasis with ascites. Hepatic stasis leads to hepatic failure with jaundice and fatty stools (steatorrhoea). · The pancreas (weight 100 g) of adult humans is capable of elaborating approximately 1.5 l of pancreatic juice daily, and its pH increases with increasing secretion rate. The maximal secretion rate is one ml per g of tissue each hour (ie, 60 times less than that of the salivary glands). · The pancreatic juice is a clear fluid, isosmolar with plasma. The basic reaction is due to bicarbonate, and the [bicarbonate] can approach the [H+] in gastric juice (150 mM). · Pancreatic acinar cells produce enzymes for digestion of carbohydrates, proteins and fats. CCK stimulates the enzyme secretion. · Acute biliary tract disease is diagnosed by biliary pain and confirmed by ultrasonographic or computer tomographic evidence of a distended/inflamed gall-bladder. The disorders are subdivided into inflammatory (acute cholecystitis) or obstructive (eg, stone in the common bile duct). Baillière´s Clinical Gastroenterology. Quarterly reviews of Gastroenterology. London: Bailliere Tindall. Gastroenterology. Monthly journal published by the Am. Gastroenterological Association, WB Saunders Co, The Curtis Center Suite 300, Independence Square West, Philadelphia , Pa 19106-3399, USA. Hug, M., C. Pahl, and I. Novak. "Effect of ATP, carbachol and other agonists on intracellular calcium activity and membrane voltage of pancreatic ducts." Pflügers Arch 426: 412-18, 1994.
|
||
Click here to introduce your comments or contributions