New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 22: Gastrointestinal Function and Disorders
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
Chapter 22
|
 |
|||||||||||||||||||||||||||||||||||||||
|
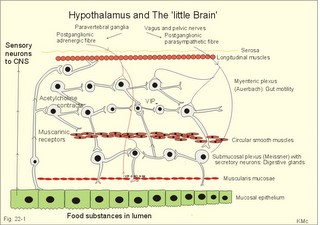
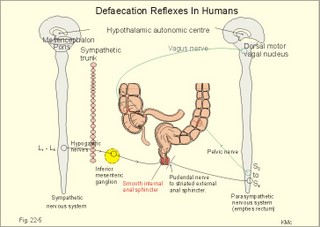
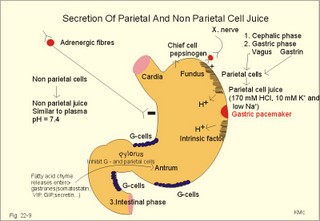
· To define concepts such as achlorhydria, enterogastrones, haematemesis, incretins, macrolide, malabsorption, melaena, migrating motor complex, paracrine secretion, peptide hormone families, peptic ulcer disease, peristalsis, segmentation, slow waves, and spike potentials. · To describe the extrinsic and intrinsic enteric nervous system including neurotransmitters and gastrointestinal hormones, cholesterol and lipid metabolism, · To explain gastrointestinal motility, gastrointestinal secretion (saliva, gastric juice, pancreatic juice, bile), digestion and intestinal absorption of nutrients, vitamins, water and iron. To explain the pathophysiology of common gastrointestinal disorders including malabsorption of carbohydrate, amino acids and fat, osmotic and secretory diarrhoea, and iron deficiency. · To use the above concepts in problem solving and case histories. · The central autonomic nervous system (hypothalamus and brain stem) mediates its influence on the gastrointestinal function through the intrinsic, enteric nervous system (the so-called “little brain”). · Cannons law of the gut: The peristalsis of the small intestine always proceeds in the oral- aboral direction. · Achlorhydria refers to absence of HCl production in the stomach · Defaecation is a reflex act involving colon, rectum, anal sphincters and many striated muscles (diaphragm, abdominal and pelvic muscles). The motor pathway is the pelvic nerves. Defaecation implies a temporal release of anal continence brought about by a reflex. The coordinating centre is in the sacral spinal cord. · Enterogastrones are enterogastric inhibitory hormones liberated from the duodenal mucosa by acid chyme (ie, cholecystokinin: CCK, gastric inhibitory peptide: GIP, secretin, somatostatin, neurotensin and vasoactive intestinal peptide: VIP). · Haematemesis is defined as vomiting of whole blood or blood clots. · Incretins are hormones, which increase insulin secretion from the b-cells of the pancreatic islets much earlier and to a greater extent, than when the blood glucose concentration is elevated by intravenous infusion (GIP, glicentin, glucagon-like peptides-1 and -2). · Intrinsic, enteric nervous system refers to the large number of neuronal connections in the gut wall, in particular the submucosal Meissner plexus, which regulates the digestive glands, and the myenteric Auerbach plexus, primarily connected with gut motility. · Macrolides are antibiotics, which bind to and prevent translocation on bacterial ribosomes. · Malabsorption describes the condition resulting from inefficient absorption of nutrients by the gastrointestinal tract. · Melaena is defined as passage of dark tarry stools (coal-black, shiny, sticky, and foul smelling). · Migrating motor complex refers to a gastric sequence of events, where contractions occur each 90 min during fasting. There is a quiet period (I) followed by a period of irregular contraction (II), and culminated with a peristaltic rush (III) accompanied by increased gastric, pancreatic and biliary secretion. · NANC neurons are non-adrenergic, non-cholinergic postganglionic neurons, which liberate gastrin-releasing peptide (GRP) to the gastrin producing G-cells. · Nitric oxide (NO) is a possible neurotransmitter between the preganglionic and the NANC postganglionic neurons. · Paracrine secretion is the release of signal molecules to neighbour cells. · Peptide hormone families are groups of hormones that exhibit sequence homology: They possess a common amino acid sequence, such as the gastrin family, which has sequence homology in their terminal penta-peptide. Peptide hormones have autocrine and paracrine functions in the gastrointestinal tract. · Peristalsis is a propagating contraction of successive sections of circular smooth muscle preceded by a dilatation. The dilatated intestinal wall is drawn over its content in this reflex mechanism, which transports the content aborally and is called the law of the gut. · Segmentation divides the small intestine into many segments by localised circular smooth muscle contractions. Segmentation mixes the intestinal content and propagate it at a slow rate, which allows sufficient time for digestion and absorption. · Slow waves (basic electrical rhythm) are slow gastrointestinal depolarisation’s occurring at a frequency of 3-18 per min. The slow waves change the resting membrane potential of smooth muscles from -50 to -40 mV. · Spike potentials are periodic fast waves of depolarisation that most often follow a slow wave, and then always initiate gastric contractions (elicited by a rise in cytosolic [Ca2+]). · Vaso-active intestinal peptide (VIP) is a vasodilatator in line with adenosine, ATP, NO. The increased bloodflow increases intestinal secretion. This paragraph deals with 1. The autonomic and enteric nervous system, 2. The cephalic, gastric and intestinal digestive phase, 3. Mastication and swallowing, 4. Gastric and intestinal motility, 5. Vomiting, 6. Colonic motility and defecation, 7. Gastrointestinal hormones, 8. Saliva, 9. Gastric secretion, and 10. Intestinal digestion and absorption. 1. The autonomic and the enteric nervous system The digestive system is innervated with nerve fibres of both the sympathetic and parasympathetic divisions, although the parasympathetic control dominates (Fig. 22-1). Movements of the gastrointestinal tract are brought about by smooth muscle activity. There is an outer longitudinal layer, an inner circular layer, and a submucosal muscle layer (muscularis mucosae) with both circular and longitudinal fibres that moves the villi of the mucosa. The inner surface is lined with mucosal epithelium (Fig. 22-1). The outer muscle layer is covered by the serosa, which is continuous with the mesentery containing blood vessels, lymph vessels and nerve fibres. The main CNS centres regulating digestive functions are located in the brain stem, where the sensory taste fibres from gustatory, tactile and olfactory receptors terminate on the cell bodies of the motor vagal and salivary nuclei. Many afferent, sensory fibres in the vagus nerve inform the central autonomic system about the condition of the gut and its content. The higher cortical and olfactory centres influence these brain stem motor centres and their parasympathetic outflow. The parasympathetic system increases digestive activity (secretion and motility), and the sympathetic system has a net inhibitory effect. The generally inhibitory digestive effects of the sympathetic nervous system are caused indirectly by vasoconstriction, which reduces bloodflow in the digestive tract. The vagus nerve innervates the gastrointestinal tract down to the transverse colon and contains both efferent and afferent fibres. The last part of the gastrointestinal tract receives parasympathetic innervation from the pelvic nerves. The efferent parasympathetic fibres enhance digestive activities by stimulating local neurons of the intrinsic, enteric nervous system located in the gut wall (Fig. 22-1). Fig. 22-1: The autonomic innervation of the gastrointestinal system and the structure of the enteric wall. – A sensory neuron to the CNS is shown to the left. The intrinsic, enteric nervous system consists of two sets of nerve plexi. The submucosal Meissner plexus mainly regulates the digestive glands, whereas the myenteric Auerbach plexus, located within the muscle layers, is primarily connected with gut motility (Fig. 22-1). The nerve plexi contain local sensory and motor neurons as well as interneurons for communication. Motor neurons in the myenteric plexus release acetylcholine and Substance P. Acetylcholine contracts smooth muscle cells, when bound to muscarinic receptors. Inhibitory motor neurons release vasoactive intestinal peptide (VIP) and nitric oxide (NO). These molecules relax smooth muscle cells. Sensory neurons are connected to mucosal chemoreceptors, which detect different chemical substances in the gut lumen, and to stretch receptors, which respond to the tension in the gut wall, caused by the food and chyme. The short effector neurons increase digestive gland secretion and induce smooth muscle contraction. The large number of neuronal connections constitutes the intrinsic, enteric nervous system, mediating brain influence on digestive functions. The enteric nervous system is also called the little brain. 2. The cephalic, gastric and intestinal digestive phase The secretion related to a meal occurs in three phases (Table 22-1). 2a. The cephalic phase is elicited even before food arrives to the stomach. The thought, smell, sight, or taste of food signals to the limbic system (including the hypothalamus) that elicits an unconditioned reflex secretion with intensity dependent upon the appetite.
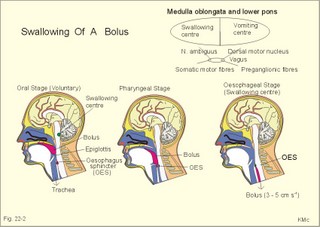
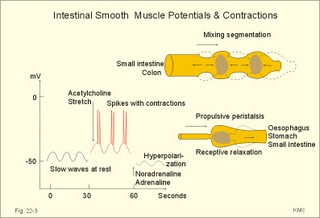
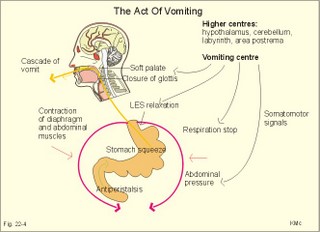
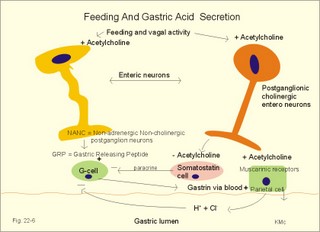
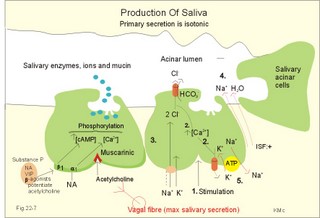
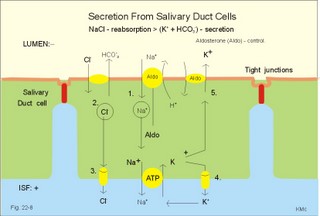
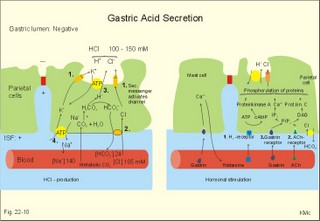
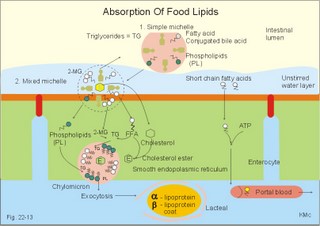
is brought about when food enters and distends the stomach. Distension stimulates stretch receptors and peptide sensitive chemoreceptors. They provide afferent signals for both long, central vago-vagal reflex loops as well as local, enteric reflexes. Signals in these fibres reach cholinergic, muscarinic receptors on the basolateral membrane of the parietal cells. Distension of the body of the stomach can release gastrin from the antral mucosa by vagal reflexes. Most of the daily gastric secretion of 1.5 l is accounted for by the gastric phase. 2c. The intestinal phase is elicited by duodenal and jejunal mechanisms that both stimulate and inhibit gastric acid secretion. Gastric secretion and motility are at first increased to promote further digestion and emptying. This fills the duodenum with acidic and fatty chyme. Acid chyme reaching the duodenum with peptides and amino acids releases gastrin from duodenal G-cells, which increases gastric secretion. Normally, the inhibitory intestinal mechanisms dominate, when the pH of the chyme is low. Acid chyme in the duodenum causes release of secretin (from S-cells) and of bulbogastrone (Table 22-1). The process of chewing or mastication requires co-ordination of the chewing muscles, the cheeks, the palate and the tongue. Chewing is normally a reflex action. The forces involved in grinding and cutting the food are enormous, and sufficient to fragment cellulose membranes. Finally, the food is mixed with saliva and formed into a bolus. The bolus is pushed back into the pharynx, when the tongue is pressed against the hard palate. Fig. 22-2: Swallowing of a food bolus in three steps (OES stands for the upper Oesophageal sphincter). The gastrointestinal tract moves ingested materials and secretions from the mouth to the anus. These movements, as well as nonpropulsive contractions, are called motility. Gastrointestinal sphincters possess adrenergic a1-receptors. Stimulation of these receptors results in contraction. Swallowing (deglutition) begins as a voluntary process by which the tongue pushes a portion of the food back against the soft palate (Fig. 22-2). Elevation of the soft palate closes the nasopharynx, and the food enters the pharynx, the larynx is elevated closing the epiglottis and respiration stops. The upper pharyngeal constrictor contracts, initiating sequential contractions of the other pharyngeal constrictors. These contraction waves are involuntary and push the food towards the oesophagus. Peristalsis in the oesophagus is started as the pharyngeal wave passes through the upper oesophageal sphincter (Fig. 22-2). When the propulsive wave reaches the lower oesophageal sphincter (LES), the relaxed muscle wall preceding the bolus momentarily relaxes the LES, and the food passes the cardia to enter the stomach. Vagal stimulation relaxes both sphincters (see achalasia, below). The upper third of the oesophagus is composed of striated muscle, the middle third contains mixed smooth and striated muscle, and the lower third contains only smooth muscle. Swallowing is controlled by brainstem neurons. They form a swallowing centre (Fig. 22-2). The vagus nerve contains both somatic motor neurons (originate in the nucleus ambiguus) that form motor endplates on striated muscle fibres, and visceral, preganglionic motor neurons (from the dorsal motor vagal nucleus to the myenteric plexus). The swallowing reflex coordinate motor signals from both oesophageal striated and smooth muscles as well as signals to the upper and lower oesophageal sphincters. Sympathetic stimulation contracts the LES mediated by noradrenaline acting on a-receptors. When a swallow is initiated via touch receptors in the pharynx, or when the lower oesophagus is distended by a bolus, it will relax the LES by reflexes in inhibitory vagal fibres joining the enteric nervous system. VIP and NO act as transmitters. 4. Gastric and intestinal motility In the stomach, digestion continues (salivary amylase) and the stomach regulates emptying of its content into the duodenum. The fundus has a high compliance, so food can accumulate without much increase in gastric pressure. Vagal fibres releasing VIP to inhibitory neurons of the myenteric plexus mediate this receptive relaxation. The body of the stomach mixes and grinds the food with gastric juice - also by retropulsion (backward or oral movement) - and then propels the content toward the antrum and pyloric region for regulated emptying. The distal stomach reduces solids to a fluid consistently composed of particles less than 2 mm. Here is a forceful peristalsis (ie, propagating contractions), so the pyloric sphincter opens and the chyme is ejected into the duodenum (Fig. 22-3). Fig. 22-3: Intestinal smooth muscle potentials (left) and contractions (right). Along the greater curvature of the stomach is a region of rapid spontaneous depolarization, which is called the gastric pacemaker establishing the maximum rate of gastric contractions. The gastric smooth muscle wall generates two types of electrical activity. Slow waves (basic electrical rhythm) are slow depolarisation’s occurring at a frequency of three in the stomach, up to 18 in the duodenum and 8 per min in the terminal ileum. The slow waves are oscillations of the resting membrane potential (Fig. 22-3). Voltage-gated (potential sensitive) Ca2+-channels open at a certain threshold of depolarization, causing a Ca2+-influx to the smooth muscle cell resulting in the so-called spikes and contractions. Spikes are periodic fast waves of depolarisation that always initiate gastric contractions, elicited by the rise in cytosolic [Ca2+]. These contractions last up till 3 s, because the Ca2+ -channels open slowly and remain open longer than the Na+ -channels. Spikes are elicited by vagal signals, by acetylcholine (muscarinic receptors), by stretch, by myenteric signals and by gastrin (Fig. 22-3). Adrenaline and noradrenaline relax smooth muscle by hyperpolarization through a-adrenergic receptors. Relaxation occurs when intracellular Ca2+ is returned to the extracellular fluid and to the endoplasmic reticulum. The small intestine is about 8 m long and commonly divided into three segments: the duodenum, jejunum and ileum. The intestinal contents must be moved in a manner that brings them into contact with the mucosa of the intestine, and propels the contents along this tubular organ. Several pacemaker regions in the small intestine control the slow waves. The pacemaker rate is highest in the duodenum (about 18 each minute), and decreases down to 8 waves each min in the terminal ileum. During fasting, a migrating sequence of events called the migrating motor complex occurs each 80-90 min. The complex consists of an 80-90 min long quiet period (I) followed by a period of irregular propulsive contractions (II), culminating in a peristaltic rush (III) to begin in the stomach, accompanied by increased gastric, pancreatic and biliary secretion. The migrating motor complex is the "intestinal housekeeper", which cleanses the digestive tract of non-absorbable substances, and provides an effective emptying of the tract all the way. During the fed state, segmentation serves to mix chyme with enzyme-containing digestive fluid, and brings the mixture into contact with the mucosal surface for absorption. Segmentation divides the small intestinal content into many segments by localised circular smooth muscle contractions with only a small propulsive effect (Fig. 22-3). Propulsive motility is accomplished by peristalsis. Peristalsis is a propagating contraction of successive sections of circular smooth muscle preceded by a dilatation (Fig. 22-3). The dilatated intestinal wall is drawn over its content in this reflex mechanism, which has been called the law of the gut. Peristaltic contractions usually travel along a small length of the small intestine, except for the peristaltic rush related to the migrating motor complex. The ileocoecal sphincter prevents retrograde flow of colonic matter. The sphincter regulates emptying of ileum five hours after a meal. The emptying of ileum is stimulated by gastrin, possibly via the gastro-ileal reflex, but a distended colon inhibits the emptying. The gastro-ileal reflex is an increased motility of the terminal ileum caused by elevated gastric activity. On the other hand, distension of the terminal ileum decreases gastric motility. The ileocoecal sphincter is normally passed by one litre of faecal matters per day. The feeling of nausea, and an array of sympathetic and parasympathetic responses initiate vomiting or emesis. Sympathetic responses include sweating, pallor, increased respiration and heart rate and dilatation of pupils. Parasympathetic responses include profuse salivation, pronounced motility of the oesophagus, stomach, and duodenum, relaxation of the oesophageal sphincters. Duodenal contents can be forced into the stomach by anti-peristalsis (Fig. 22-4). During the expulsion of gastric contents, the person takes a deep breath, the pylorus is closed, the glottis is closed so respiration stops, and the stomach is squeezed between the diaphragm and the abdominal muscles, causing rapid emptying (Fig. 22-4). Vomiting is co-ordinated by the vomiting centre in the medulla. Fig. 22-4: Vomiting co-ordinated by the vomiting centre. Vomiting is stimulated in certain areas of the brain (hypothalamus) and the cerebellum through sensory stimuli or injury. Vomiting is also provoked by certain labyrinthine signals, and from the chemoreceptive trigger zone located on the floor of the 4th ventricle close to area postrema. During deep anaesthesia the vomiting and swallowing mechanisms are paralysed. Any patient must abstain from food and water for at least six hours before deep anaesthesia is administered. Otherwise, the patient may vomit into the pharynx, and suck his own vomit into the trachea. Over the years, many patients have choked to death due to this mechanism. The survivors develop aspiration pneumonia. Such events are clearly malpractice. The swallowing mechanism is also cut-off by injury of the 5th, 9th, or 10th cranial nerve, by poliomyelitis, by myasthenia gravis and by botulism (Chapter 33). An acute loss of H+ from the extracellular fluid (ECF) by vomiting creates a metabolic alkalosis (high pH with high Base Excess, see Chapter 17). 6. Colonic motility and defaecation Colonic transit is measured in days. Mixing occurs in the ascending colon, because peristalsis is followed by anti-peristalsis. Slow waves of contraction move the content in the oral direction to delay propulsion and increase absorption of water and electrolytes. Colonic segmentation is a mixing of the content by regular segments called haustrae. Prominent haustration along the length of the colon is characteristic for the X-ray image of the normal colon. The colon provides an optimal environment for bacterial growth. Peristaltic rushes in the colon occur several times per day. They often start in the transverse colon as a tight ring, continuing as a long contraction wave. Gastro-colic and duodeno-colic reflexes assisted by gastrin and by cholecystokinin (CCK) promote peristaltic rushes. Defaecation is a complex act involving both voluntary and reflex actions in colon, rectum, anal sphincters and many striated muscles (diaphragm, abdominal and pelvic muscles). Defaecation is a temporal release of anal continence brought about by a reflex. The rectum is usually empty, and its wall has a rich sensory supply. Distension of the recto-sigmoid region with faecal matter releases awareness of the urge to defaecate, an intrinsic defaecation reflex, and a strong, spinal reflex. There is a reflex contraction of the descending colon and the recto-sigmoideum. The smooth internal anal sphincter muscle maintains a tonic contraction during continence, due to its sympathetic fibres from the lumbar medulla (through hypogastric nerves and the inferior mesenteric ganglion). The muscle relaxes due to its parasympathetic, cholinergic fibres in the pelvic splancnic nerves (S2-S4). The strong spinal reflex produces relaxation of the smooth muscles of the internal anal sphincter (Fig. 22-5) and contraction of the striated muscles of the external anal sphincter (innervated by somatic fibres in the pudendal nerve) inhibiting the reflex and causing receptive relaxation. This is the last decision - before defaecation. Fig. 22-5: Defaecation reflexes. The levator ani muscle contributes to the closure of anus, because contractions increase the angle between the rectum and the anus. Destruction of the lower sacral medulla (the defaecation centre) destroys the spinal reflex and thus the normal defecation. Higher spinal lesions destroy the voluntary control, whereas the defaecation reflexes persist. An acceptable status is obtainable in paraplegics by mechanical release of the reflex (manual expansion of the external sphincter) once daily following a meal. Gastrointestinal hormones are peptides secreted by the gastrointestinal mucosa, and controlling all gastrointestinal functions together with other hormones and transmitters. As an example insulin works together with acetylcholine and parasympathomimetics to stimulate secretion and motility, whereas catecholamines, sympatomimetics and parasympatolytics, such as atropine, inhibit gastrointestinal secretion and motility. Peptide hormone families are groups of regulatory peptides that exhibit sequence homology (ie, they possess a common amino acid sequence). The gastrin-family and the secretin-glucagon family are the most important. consists of gastrin and cholecystokinin (CCK) in three different forms (CCK-8, CCK-22, and CCK-33). Gastrin and CCK release pancreatic glucagon from the islet cells. There are two major forms of gastrin in the plasma, normal gastrin or G-17 and big gastrin or G-34. They are 17 and 34 amino acid polypeptides, respectively. Gastrin is produced by G-cells of the gastric antrum and duodenum. The duodenal Brunner glands secrete half of the G-34. Gastrin is the strongest stimulator of gastric acid secretion. Gastrin also imposes tropic (growth-stimulating) actions on the parietal cells, the mucosa of the small and large intestine and possibly the pancreas. Gastrin stimulates the pepsin secretion from peptic cells, and the glucagon secretion from the a-cells of the pancreatic islets. Gastrin is derived from parietal or oxyntic cells in the stomach. When stimulating gastric acidity, gastrin relaxes the gastric muscles, thus retarding the passage of chyme into the duodenum. Feeding induces the secretion of gastrin to the interstitial fluid and then to the blood. Neural signals pass through the vagal nerve to the gastrin-secreting G-cells of the gastric antrum and duodenum (Fig. 22-6). The afferent input begins with the smell and taste of food, and is reinforced by vago-vagal reflexes elicited by oesophageal and gastric distension. Digested protein (polypeptides and amino acids) act directly on G-cells. Fig. 22-6: Gastric HCl secretion following feeding. GRP: Gastrin Releasing Peptide. NANC: Non-adrenergic, Non-cholinergic postganglionic neurons. Vagal, cholinergic preganglionic fibres transfer signals to the G-cells via non-adrenergic, non-cholinergic (NANC) postganglionic neurons. These enteric neurons liberate gastrin-releasing peptide (GRP) to the gastrin producing G-cells. The gastrin released reaches the parietal cells through the blood and increases the HCl secretion. GRP thus releases gastrin and hereby stimulates the secretion of gastric acid. - GRP consists of 27 amino acid moieties and is also released from neurons in the brain. An indirect vagal route to the G-cells is via postganglionic cholinergic enteric neurons to somatostatin cells that are located close to the G-cells (Fig. 22-6). When these enteric neurons release acetylcholine, the response of the somatostatin cells is inhibition of somatostatin release. Somatostatin inhibits G-cell secretion by paracrine action. The result of both vagal inputs to the G-cells is gastrin release (Fig. 22-6). An elevated [H+] in the duodenal lumen inhibits gastrin release. Cholecystokinin, CCK, according to its function and structure, belongs to the gastrin family. Cholecystokinin empties the gall bladder as the name implies, and stimulates pancreatic secretion of an enzyme rich juice. However, CCK has a higher affinity for receptors stimulating gallbladder contraction and pancreatic enzyme secretion. CCK has a maximal effect only in the presence of secretin (potentiation) and normal vagal influence. Both gastrin and CCK release glucagon from the a-cells of the pancreatic islets. CCK is cleaved from pre-pro-CCK in the duodenum, upper jejunum (I-cells) and in the brain. CCK molecules consist of a group of peptides. CCK-8, CCK-22 and CCK 33 are the dominant forms in the blood. The most important stimulus for CCK liberation is amino acids and fatty acids, which reach the duodenal mucosa. Bile is ejected into the duodenum, where fat is emulgated to ease its absorption. CCK also acts as an enterogastrone - an intestinal hormone that inhibits gastric activity and emptying. This leaves more time for the bile to emulgate fat. 7 b. The secretin-glucagon family Secretin exhibits sequence homology with pancreatic glucagon, vasoactive intestinal peptide (VIP), growth hormone-releasing hormone (GHRH) and gastric inhibitory polypeptide (GIP). A family of five genes code for these five hormones. Secretin is secreted by S-cells in the mucosa of the upper small intestine, when acid chyme (pH below 4.5) arrives to the first part of the duodenum. Fatty acids from fat digestion also contribute to secretin release. Secretin stimulates the secretion of bicarbonate and water by pancreatic duct cells, and of bicarbonate-rich aqueous bile. Secretin potentiates the action of CCK including an enterogastrone effect (gastric inhibiting effect). Secretin antagonises gastrin - and potentiates CCK. Secretin is an enterogastrone that is released by H+ to stimulate pancreatic juice secretion. Gastric inhibitory polypeptide (GIP or Glucose-dependent Insulin releasing peptide) works as the two names imply: GIP inhibits the gastric mucosa and releases insulin from the a-cells of the pancreatic islets. Glucagon is actually two different molecules: Intestinal glucagon (glicentin) and pancreatic glucagon. Both are hepatic insulin-antagonists. Glucagon stimulate glycogenolysis, gluconeogenesis (urea genesis- glycogenic amino acids), and ketogenesis. The function of other peptide hormones is given in Table 22-2.
Saliva is a watery solution of electrolytes (bicarbonate and K+) and organic substances, which is a mixture of secretions from three pairs of glands. The parotid is the largest and serous (watery saliva), the sublingual is mucous (viscous, containing mucin), and the submandibular salivary gland is build of mucous acini surrounded by serous half moons. The primary saliva is produced in the acini, but secondary processes in the salivary ducts (secretion and reabsorption) are involved in the final saliva production. Salivary glands have a high bloodflow and produce up to one l of saliva daily. The maximal secretion rate is one ml of saliva per g salivary tissue per min (ie, 60 times that of pancreas). Salivary mucin (a glycoprotein) and water lubricate food, dissolve particles, and salivary enzymes initiate digestion. Ptyalin or a-amylase cleaves a-1-4 glycoside bindings in starch. Salivary buffers maintain the pH-optimum (6.8) of amylase during the first period in the stomach. The saliva dilutes injurious agents. Saliva cleans the mouth and pharynx (prevents caries), and ease swallowing. Salivary lysozyme lyses bacterial cell walls. The salivary epidermal growth factor promotes the healing of wounds. Animals instinctively lick their wounds. Saliva contains immuno-defensive secretory globulin A (IgA), amino acids, urea, and blood-type antigens in secreting persons. Saliva may inactivate human immunoactive virus (HIV). The most common infection of the salivary glands is acute parotitis caused by the mumps virus. The virus causing infectious mononucleosis is probably transferred with saliva by "deep kissing". Infectious mononucleosis is a disease characterised by lympadenopathy, lympho-cytosis and duration longer than an ordinary tonsillitis. The condition is dangerous, because spontaneous rupture of the spleen occurs. Salivary secretion is controlled by the autonomic nervous system, and minimally influenced by hormones. Unconditioned reflexes (taste-, olfactory- and mechano-receptors) control salivation as well as conditioned reflexes (the thought of food). These signals reach the brain stem salivary centres, which activate the parasympathetic nerves to the salivary glands. The primary salivary secretion into the acini resembles an ultrafiltrate of plasma, but the final saliva is hypotonic. Parasympathetic, cholinergic fibres, originating in the salivary nuclei of the brain stem, synapse with postganglionic neurons close to the secretory cells. These neurons transmit signals to the cholinergic, muscarinic receptors (Fig. 22-7). Parasympathetic activity can release maximal salivary secretion and bloodflow resulting in a amylase-rich saliva with mucin (glycoproteins). Atropine blocks the muscarinic, cholinergic receptors (during anaesthesia where the mouth becomes dry). The rise in bloodflow is atropine-resistant and caused by the vasodilatating VIP, which is released from peptidergic nerve terminals that also contain acetylcholine. b1-adrenergic agonists and VIP elevate cAMP in the acinar cells, an effect potentiating the secretory effect of acetylcholine. The vascular smooth muscle relaxation by VIP is probably also mediated via cAMP. Fig. 22-7: Salivary enzymes, ions and mucin production from two acinar cells. Solid and dashed arrows indicate active and passive transport, respectively. Circles are carrier molecules, whereas tubes symbolise transport channels. - To the left is shown receptors and second messengers. 1. Neural or humoral (acetylcholine) stimulation of cholinergic, muscarinic receptors on the basolateral membrane of acinar cells leads to a rise in intracellular [Ca2+]. 2. This rise triggers luminal Cl- - and basolateral K+ -channels. Hereby, K+ is transferred to ISF and Cl- to the acinar lumen in a balanced relationship (Fig. 22-7). Therefore, Cl- flows down its electrochemical potential gradient into the lumen of the acinus. K+ flows down its gradient to the ISF through activated channels. These ion flows create a negative electric field in the lumen. 3. The initial fall in intracellular [K+] increases the driving force of the electroneutral Na+-K+-2Cl- co-transporter to transport two Cl- into the cell together with Na+ and K+. Thus the electrochemical potential of Cl- and K+ is greater in the cell, than in the interstitial fluid (ISF) and in the saliva. 4. The negative field provides an electric force that drives a passive Na+ flux into the acinar lumen through leaky tight junctions. Osmotic water transport through leaky junctions and trans-cellularly through water channels in the cell membranes follow the NaCl flux into the lumen. The trans-cellular Cl- transport is coupled to the paracellular Na+ transport. The net result is an isosmotic NaCl transport produced by a secondary active Cl-- secretion. 5. The basolateral membranes of acinar cells contain a Na+-K+-pump that provides the energy for the primary salivary secretion (Fig. 22-7). The rise in intracellular [Na+] from 2., activates the Na+-K+-pump, whereby [Na+] is kept almost constant. Ouabain inhibits salivary secretion, because it blocks the pump. Sympathetic nerve signals, and circulating catecholamines via b-adrenergic receptors, inhibit the bloodflow and the secretion of serous saliva (b1-receptors in Fig. 22-7). A small, transient, mucous secretion with a high [K+] and [bicarbonate], and a low [Na+] is produced, because of the low secretion rate. Noradrenaline (NA) stimulates both a1-adrenergic and b1-adrenergic receptors. Binding of NA or b-adrenergic agonists elevates intracellular cAMP, which correlates with a small increase in primary salivary secretion. This explains why the mouth becomes dry during events, where the sympathetic system dominates (anxiety, excitement etc). The salivary ducts are almost watertight. Therefore, the final salivary flow is dependent upon the primary salivary secretion rate in the acini. The duct systems, in particular the small-striated ducts with a substantial O2 consumption reabsorb large amounts of Na+ and Cl-, whereas bicarbonate and K+ are secreted. Saliva becomes more and more hypotonic at low secretion rates, because the Na+ and Cl- reabsorption dominate. 1. The reabsorption of Na+ and the secretion of K+ are processes stimulated by the mineralo-corticoid, aldosterone. Aldosterone stimulates Na+-influx through the luminal Na+-H+-exchanger (Fig. 22-8). Na+ enters the cell in exchange with H+. The resulting intracellular rise in [Na+] activates the basolateral Na+-K+-pump. Thus, Na+ is reabsorbed trans-cellularly from the salivary duct. The pump maintains the electrochemical potential gradients of Na+ and K+. 2. The Cl- follows passively, and is partly exchanged with bicarbonate along the duct system through a luminal Cl- - bicarbonate exchanger (Fig. 22-8). The secretion of bicarbonate is so great that its concentration in the final saliva exceeds that in plasma. 3. At the basolateral membrane Cl- leaves the cell via an electrogenic Cl- channel, while Na+ is pumped out. 4. K+, taken up by the Na+-K+-pump, leaves the cell through K+-channels in the basolateral membrane, recycling K+ to balance the Cl- efflux. 5. Some of the K+ leaves the cell by luminal H+-K+-exchange. At low secretion rates the H+-K+-exchanger (antiport) in the luminal membrane transfers sufficient K+ for the [K+] in the final saliva to exceed the concentration in plasma. The net result is K+-secretion from blood to the duct lumen. The final salivary [Na+] and [Cl-] increase with increasing salivary secretion rate, because the high flow provides less time for reabsorption in the duct system. Bicarbonate may be secreted even without Cl--reabsorption. At low salivary secretion rates the final saliva becomes hypotonic down toward half of the osmolarity of plasma. Fig. 22-8: Secretion from salivary duct cells. The aldosterone effects described above (increased Na+ reabsorption and increased K+ secretion) are similar to those in the distal, renal tubules and in the sweat glands. The stomach is divided into three main regions: the fundus, corpus and pyloric antrum. The gastric mucosa is highly invaginated and is mainly composed of gastric glands, with mucous neck cells, parietal cells secreting HCl, and peptic (chief) cells secreting pepsinogen. The parietal cells also secrete the peptide intrinsic factor, which is necessary for absorption of vitamin-B12. G-cells in the mucosa produce the hormone gastrin (Fig. 22-6). The gastric secretions include hydrochloric acid (HCl), pepsin and basic mucus, which contains mucin (glycoproteins) and salts. Efferent signals from the dorsal motor nuclei of the vagi stimulate gastric motility and HCl production. Acetylcholine is released from the short postganglionic vagal fibres and directly stimulates parietal cells to secrete HCl. The parietal cells contain muscarinic receptors on the basolateral membrane. Vagal fibres work together with intrinsic, peptidergic neurons containing vasoactive intestinal peptide (VIP) and gastrin releasing peptide (GRP). VIP controls the bloodflow of the gastric mucosa; GRP releases the important gastrin from the antral G cells and the peptic cells secrete pepsinogen. The secretion related to a meal occurs in three phases (cephalic, gastric and intestinal). The gastric juice is hyperosmotic (325 mOsmol/l), contains 10 mM of K+ and is low in Na+ at moderate and high secretion rates; the [H+] is 170 mM and the [Cl-] is 180 mM. Gastric juice has an approximate pH of 1, forming a million-fold gradient of H+ across the gastric mucosa to the blood. The HCl activates pepsinogen, maintains the optimal pH for pepsin activity and denatures proteins and microbes. The peptic cells, located in the base of the gastric gland, produce pepsinogen. Pepsinogen is stored in granules of the peptic cell. Pepsinogen secretion is stimulated by cholinergic, muscarinic substances and by b-adrenergic agents, but peptic cells have no histamine receptors. Exocytosis releases pepsinogen into the gastric juice, where it is cleaved into pepsin, if HCl is present. Pepsin is the major hydrolytic enzyme in the stomach, but it is only active in the acidic gastric juice. Fig. 22-9: Secretion of parietal and non- parietal cell juice. Adult humans produce up to two l of gastric juice daily. The gastric juice is produced from two different sources: The parietal cell juice with 170 mM [HCl], 10 mM [K+], and a low [Na+]. A juice with an ionic composition similar to that of plasma is produced from other cells - the non parietal juice. Each of the two secretion products has almost a constant composition. Increased secretion of gastric juice means increased secretion of parietal cell juice. This explains why the [HCl] increases more and more in the mixed product, whereas [Na+] falls with increasing secretion rate. Fatty chyme entering the duodenum delays gastric emptying by negative feedback through duodenal reflexes and by the release of gut inhibiting hormones (so-called enterogastrones: somatostatin, VIP, gastric inhibitory peptide, GIP, neurotensin and secretin). These inhibitors not only inhibit gastric motility; they also inhibit the gastrin release from the antral G cells, and also the HCl production from the parietal cells. Mucus contains mucin (glycoproteins) and electrolytes with bicarbonate that protect the gastric mucosa from adversive effects. Stimulation of the parietal cells with acetylcholine, histamine and gastrin has two consequences for their content of second messengers (Fig. 22-10, right). The cellular [Ca2+] and [cAMP] is elevated. Fig. 22-10: HCl secretion from parietal cell in the stomach (left). Secretory receptors on the parietal cell are also shown (right). 1. These second messengers activate luminal Cl-- and K+-channels. Cl- and K+ pass into the lumen, whereby their cellular concentrations decrease (Fig. 22-10 left). The luminal [K+] activates the K+-H+-pump. In addition, more pumps are inserted into the luminal membrane from cellular tubulo-vesicles. 2. The fall in cellular [Cl-], and a rise -see below - in cellular [bicarbonate], stimulates the basolateral Cl--bicarbonate exchanger, whereby the cellular [bicarbonate] is reduced. The fall in cellular [H+] and [bicarbonate] stimulates formation of H+ and bicarbonate, under the influence of carbo-anhydrase (*). The H+ and bicarbonate are derived from metabolic carbon dioxide from the blood. Bicarbonate diffuses from the interstitial fluid space (ISF) into the blood. Every time the gastric juice receives one H+, the blood will receive one HCO3-. This explains why the pH of the gastric venous blood increases after a meal - the alkaline tide. 3. Cellular [H+] is a substrate for the luminal gastric proton pump (the K+-H+-pump), already activated by K+. The net result is H+-secretion to the lumen in a balanced relationship to Cl--secretion. The surface of the gastric mucosa is always electrically negative with respect to the serosa. H+ moves against a large concentration gradient into the gastric lumen. The intracellular [H+] of the parietal cells is 10-7 mol/l, so with a [H+] of 10-1 mol/l in the gastric juice, a million-fold concentration gradient is present across the luminal membrane. Accordingly, energy is required for the transport of both ions. The HCl secretion requires ATP. 4. The cellular concentration of cations is maintained by the basolateral Na+-K+-pump. The parietal cells contain more mitochondrial mass per volume unit than any other cells in the body, indicating a rich oxidative metabolism. Histamine, acetylcholine and gastrin stimulate acid secretion. We have two types of histamine receptors in the human body: H1 receptors (blocked by diphenhydramine) and H2 receptors. Only H2 receptors are located on the parietal cells. 1. The H2 receptors make histamine a potent stimulant of HCl secretion. When histamine is bound to the H2 receptor it activates adenylcyclase, an enzyme generating cAMP from ATP. This increase in intracellular [cAMP] is specific for histamine. The cAMP binds to and activates cAMP-dependent protein kinase (consisting of a regulatory and an active catalytic subunit). The cAMP binding releases the active catalytic subunit, which phosphorylate a variety of target proteins. H2 receptor antagonists (cimetidine and ranitidine) prevent histamine from binding to the H2 receptors of the basolateral membrane of the parietal cells, which reduces acid secretion. Synthetic analogues of prostaglandin E can inhibit both the cAMP and the Ca2+ release mechanisms, thus promoting ulcer healing (see later). 2. Acetylcholine (ACh) is released by vagal stimulation that leads to a stimulation of acid secretion. This secretion is inhibited by atropine. Thus the parietal cells contain muscarinic, cholinergic receptors (M3). 3. Gastrin is the most potent stimulant of acid secretion in humans. Gastrin receptors were previously supposed not to be present on human parietal cells. Gastrin from G-cells was thought to release histamine from the granules of the mast cells in the gastric glands (Fig. 22-10). This is probably not the case. A direct gastrin effect on human gastrin receptors occurs, and an additional indirect effect via histamine increases the HCl secretion markedly (H2 receptors). However, the three-receptor hypothesis is still under debate. Gastrin and acetylcholine release inositol-triphosphate (IP3), which is produced with diacylglycerol (DAG) by a membrane phospholipase. The target system for IP3 is a Ca2+-channel protein located in the endoplasmic reticulum. Ca2+ is released from the reticulum, and Ca2+ also enters the cell through the basolateral membrane. Combined stimulation of all three receptors results in maximal gastric secretion (potentiation). 10. Intestinal digestion and absorption Almost all of the dietary nutrients, water and electrolytes that enter the upper small intestine are absorbed. The small intestine, with its epithelial folds, villi, and microvilli, has an internal surface area of 200 m2. Carbohydrates are the most important energy-containing components of the diet. The energetic value of most carbohydrates is 17.5 kJ per g, so that a daily diet of 400 g carbohydrates covers 7 000 kJ, which is 56% of the usable energy in a diet of 12 500 kJ daily. The formation of metabolic water on a mixed diet is 0.032 g of water per J. Fig. 22-11: Absorption of carbohydrates by the enterocyte. The common sources of digestible carbohydrates are starches (amylose), table sugar, fruits and milk. Plant and animal starch (amylopectin and glycogen) are branched molecules of glucose monomers. Indigestible carbohydrates are present in vegetables, fruits and grains (cellulose, hemicellulose, pectin) and in legumes (raffinose). Indigestible carbohydrates are also referred to as dietary fibres. Digestion of starches to simple hexoses occurs in two phases: The luminal phase begins in the mouth with the action of salivary amylase (ptyalin), but most of this phase occurs in the upper small intestine as pancreatic a-amylase reach the chyme. The starch polymer is reduced to maltose, maltriose and a-limit dextran or dextrins (Fig. 22-11). The three substrates are pushed through the intestine and are now ready for the brush-border phase. Some of the substrate molecules get into contact with the brush-borders of the absorbing mucosal cell via the unstirred water layer. Enterocytes carry disaccharidases and trisaccharidases (oligosaccharidases) on their surface that cleave these substrates to glucose, G. Milk sugar (lactose) and cane sugar (sucrose) only require a brush-border phase of digestion, since they are disaccharides. Sucrose is reduced to glucose and fructose (G-F), and lactose to glucose and galactose (G-Ga) by the action of disaccharidases (sucrase and lactase). Glucose in the intestinal lumen is absorbed by active transport. 1. The mechanism of active glucose transport is a carrier-mediated, Na+ - glucose cotransport. As the luminal [glucose] falls below the fasting blood [glucose], active glucose transport becomes essential and sequesters all remaining luminal glucose into the blood. Glucose and Na+ bind to apical membrane transport proteins (a glucose-transporter, GLUT 5). The two substances are deposited in the cytoplasm, because of conformational changes in GLUT 5, whereby the affinity of GLUT 5 for glucose-Na+ changes from high to low. Glucose accumulates inside the cell to a level that exceeds blood [glucose]. 2. Glucose therefore diffuses down its concentration gradient, through a specific uniport carrier in the basolateral membrane, out into the interstitial space and into the blood (Fig. 22-11). The basolateral uniport carrier for glucose is highly specific (glucose only), and does not depend upon Na+. Galactose is also actively transported by the luminal glucose carrier system, and is a competitive inhibitor of glucose transport. Phlorrhizin blocks the glucose absorption, when its glucose moiety binds to the transporter instead of glucose. 3. Cytoplasmic Na+ is actively pumped out through the basolateral membrane by the Na+-K+-pump. The low intracellular [Na+] creates the Na+ gradient and energises the transport of hexoses over the luminal enterocyte membrane. 4. Fructose has no effect on the absorption of glucose and galactose. Fructose is not actively transported by the Enterocytes, but is absorbed by a carrier-mediated, facilitated diffusion system, where energy is not required. The typical Western diet contains 100 g of protein, which is equivalent to an energy input of 1700 kJ daily, although an adult needs only less than one g pr kg of body weight. This luxury combustion is an inappropriate use of global resources. Moreover, a high protein intake implies a long-term risk of uric acid accumulation from purine degradation (Chapter 20). Meats, fish, eggs, and diary products are high in proteins and expensive. Vegetable proteins are not as expensive as animal proteins. Residents of areas with carbohydrate dominated nutrition and protein hunger develops diseases of protein deficiency, such as Kwashiorkor (Chapter 20). Digestion of dietary proteins begins in the stomach, with the action of the gastric enzyme pepsin (pH optimum is 1), which cleaves proteins to proteoses, peptones and polypeptides. Pepsin is produced from pepsinogen in the presence of HCl. Pepsinogen is secreted by the gastric chief cells. The digestion is continued in the intestine by proteolytic enzymes of the pancreas. Enteropeptidase converts trypsinogen to trypsin. Trypsin acts auto-catalytically to activate trypsinogen, and also convert chymo-trypsinogen, pro-carboxy-peptidases A/B, and pro-elastase to their active form. When the chyme is pushed into the duodenum, the pancreatic juice neutralises the chyme and the activity of pepsin is stopped. The proteolysis in the small intestine plays the major role, because the digestion and absorption of dietary protein is not impaired by total absence of pepsin. Cytosolic peptidases from the enterocytes and brush border peptidases from the brush borders of the villous cells then cleave the small peptides into single amino acids (Enteropeptidase, amino-polypeptidase and di-peptidases). The end products of protein digestion by pancreatic proteases and brush border peptidases are di- and tri-peptides and amino acids. The cytosolic peptidases are abundant and particularly active against di- and tri-peptides. Hydrolytic digestive products such as tripeptides, dipeptides and amino acids can be absorbed intact across the intestinal mucosa and into the blood. Two transport routes are dominant: 1. A peptide transporter, with high affinity for di- and tri-peptides, is absorbing the small peptides (Fig. 22-12). The system is stereospecific and prefers peptides of physiologic L-amino acids. This peptide transport across the brush border membrane is a secondary active process powered by the electrochemical potential difference of Na+ across the membrane. The total amount of each amino acid that enters the enterocytes in the form of small peptides is considerably greater than the amount that enters as single amino acids. 2. The absorption of single amino acids from the intestinal lumen is an active process that involves a Na+-dependent, carrier-mediated cotransport system similar to that for glucose. Competitive inhibition, saturation kinetics, Na+ dependency, and expenditure of metabolic energy in this case also characterise active transport. Selective carrier systems appear to be present for certain groups of amino acids: neutral, acidic, imino and basic groups. The neutral brush border (NBB) system transports most of the neutral amino acids. The imino acid system handles proline and hydroxyproline. Fig. 22-12: Absorption of peptides and single amino acids by the enterocyte. Basic amino acids and phenylalanine are absorbed primarily through facilitated diffusion from the gut lumen to the blood. The basolateral membrane is more permeable to amino acids than is the brush border membrane. Therefore diffusion is more important for the basolateral transport, especially for amino acids with hydrophobic side chains. The amino acids are carried in the blood to the liver via the portal vein. Half of the amino acids absorbed in the intestine are from the diet, the remaining part is from digestive secretions and from desquamated mucosal cells. Only 1 % of the dietary protein is excreted in the faeces, the remaining faecal protein is derived from micro-organisms and desquamated cells. The reabsorption of amino acids (and glucose) in the renal tubules bares many similarities to the active absorption mechanism in the intestine. A rare genetic disease involves defective intestinal absorption of neutral amino acids and a similar defective renal reabsorption. This condition is called Hartnups disease, which is caused by defects in the NBB transport system of the brush border coated epithelial cells of the jejunum and the proximal renal tubules. The typical Western diet contains 100 g of lipids (3900 kJ) daily. Most of the dietary lipids consumed are triglycerides (only 2-4% is made up of phospholipids, cholesterol, cholesterol esters etc). Lipids would comprise just above 30% (ie, 100 g = 3900 kJ) of a standard diet of 12 500 kJ daily. An optimal diet should contain only 20% lipids, such as the lipids of fish oil and olive oil. Absorption of excess lipids results in accumulation (obesity). The consequences of long term obesity are described in relation to diabetes mellitus in Chapter 27. Essential dietary fatty acids are poly-unsaturated and cannot be synthesized in the body (linoleic acid, linolenic acid and arachidonic acid). Dietary triglycerides are broken down into simpler molecules, to facilite absorption. A small fraction of the triglycerides is digested in the mouth and stomach by salivary, lingual lipase. Most dietary triglycerides (TG) are digested in the small intestine. However, two problems must be solved before digestion can occur. Triglycerides are insoluble in water, and the chyme in the intestine is an emulsion of large fat particles in water. All the lipase proteins by contrast are water-soluble. It follows that, triglycerides must be dissolved in the aqueous phase before they can be digested. The lipolytic activity requires the emulsifying action of bile salts in order to dissolve triglycerides in water. Pancreatic lipase binds to the surface of the small emulsion particles. 1. Simple bile micelles are aggregates of bile salt monomers that form spherical structures with a diameter of 5 nm, and the micelles have a negative charge. Following a meal, bile micelles are formed above a certain concentration of bile salts, called the critical micellar concentration. The lipophilic, hydrophobic, apolar end of the bile acids faces inward creating a hydrophobic core (Fig. 22-13). The hydrophilic polar end of the bile salts (hydroxyl-, carboxyl- and amino- groups) points outward, so that they are mixed with the polar water molecules. The simple lipids must pass a diffusion barrier - an unstirred water layer, which is the water layer immediately adjacent to the mucosa, where the intestinal flow rate is essentially zero. This water layer contains the water-soluble lipases and cholesterol esterases. Fig. 22-13: Absorption of lipids by the enterocyte (2-MG is 2-monoglyceride). 2. Mixed micelles. Simple lipid molecules (cholesterol, phospholipids, fatty acids, 2-monoglycerides or 2-MG, fat-soluble vitamins and lyso-lecithin) diffuse into the lipophilic core of the simple bile micelles and form a mixed micelle (Fig. 22-13). A solution of micelles is water-clear and stable. The mixed micelles carry the major part of all the lipids that are absorbed by the intestinal microvilli. When the lipids of the mixed micelle have diffused into the enterocyte, emulsifying more hydrolysed lipids recycles the empty bile micelle. Neither bile salt micelles nor bile salt molecules diffuse into the enterocyte (Fig. 22-13). The fatty acids with a short chain (up to 12 C-atoms) are more hydrophilic than the rest. They can diffuse directly to the portal blood as fatty acids. Once fatty acids enter the enterocyte, they are primarily activated to acetyl coenzyme A by a process that requires ATP and acetyl coenzyme A synthetase. Acetyl coenzyme A enters one of two pathways: the 2-MG and the a-glycerol phosphate pathways. Both bring about the resynthesis of triglycerides (TG) in the enterocyte. In the enterocyte the lipids are reformed to triglycerides, cholesterol, phospholipids etc. The reformed triglycerides, cholesterol, phospholipids, fatty acids, esters and fat-soluble vitamins reach the endoplasmic reticulum, where they are packed in another lipid-carrying particle: the chylomicron. The centre of the chylomicron is a cholesterol ester (E in Fig. 22-13). Chylomicrons are packed into vesicles in the Golgi-system. These vesicles reach the basolateral membrane, and their contents pass through this membrane by exocytosis. Thus the chylomicrons reach the lymphatic channel of the villus (the central lacteal). The lymph delivers the chylomicrons to the blood through the thoracic duct. Plasma is milky (lipaemic) following a fatty meal. All of the dietary lipid is normally absorbed in the intestine. Faecal fat derives from bacterial lipids and lipids of desquamated mucosal cells. - Disorders such as gallstones, pancreatitis, Crohn's disease, and liver disease can lead to fat malabsorption (steatorrhoea or fat-diarrhoea). Lipids are mainly absorbed through the enterocyte and transported by the lymph, which reaches the blood via the thoracic duct. Lipids thus reach the liver through the hepatic artery, with the exception of short-chain fatty acids that enter the portal blood directly. Other nutrients are absorbed directly to the blood and reach the liver through the portal vein. Fat-soluble vitamins, such as vitamin A, D and K, are absorbed in the chylomicrons along with lipid nutrients (Fig. 22-13). In contrast, the water-soluble vitamins, such as vitamin- B and -C, cross the mucosa by diffusion and by association to specific membrane transporter proteins. Vitamin B12 (cyanocobalamine) is the largest of the vitamins, and its absorption in the terminal ileum utilises a specific transport mucoprotein called intrinsic factor. The intestinal content is isosmolar with plasma, and the water is absorbed from the lumen to the blood by passive osmosis. The membranes of the intestinal mucosal cells and even the tight junctions are highly permeable to water. Hereby, active transport of Na+ and Cl- from the lumen to the small interstitial space builds up a forceful osmotic gradient, drawing water the same way by a passive process. In the small interstitial space water creates a hydrostatic overpressure. Since the capillary and lymph endothelial membranes are no barriers for Na+, Cl- and water, a bulk flow of fluid from the interstitial space passes into the blood- and lymph vessels. The intestinal mucosa possesses elevations called villi, and pitted areas called crypts. The villous cells have a typical brush border responsible for net absorption of ions and water, whereas the crypt cells contain secretory mechanisms causing net secretion. The villous cells absorb Na+ through the luminal brush border membrane by three mechanisms: 1. An inward diffusion gradient through a Na+-channel, 2. A Na+-H+-exchange, and 3. A Na+ -solute coupled cotransport (the solute being glucose, galactose, bile salts, water-soluble vitamins and amino acids). Fig. 22-14: Ion transport processes in jejunal enterocyte. Ad 1.: The [Na+] is kept low (14 mM) in the cell, whereas [Na+] is 140 mM in the intestinal lumen. This concentration gradient work together with an electrical gradient, since the cytosol of the cell is -40 mV with the intestinal content as a reference (Fig. 22-14). Thus Na+ can easily pass the luminal brush border membrane passively. The intestinal mucosa has ion permeable tight junctions - it is leaky. This paracellular transport is so great that the net absorption of Na+ and Cl- through the cells only amounts to 10% of the total transport through the mucosa. Ad 2.: The transport of Na+ into the enterocyte (Fig. 22-14) is through a co-exchange protein (Na+/H+). Part of the energy released by Na+ moving down its gradient is used to extrude H+ into the intestinal lumen. Here H+ reacts with bicarbonate from bile and pancreatic juice to produce CO2 and water, thus reducing the pH of the intestinal fluid. Ad 3.: Na+ -solute coupled cotransport. The basolateral membrane of the enterocyte contains a Na+-K+-pump, which maintains the inward directed Na+-gradient. The pump is energised by the hydrolysis of ATP, which provides the driving force for Na+ entry. Thus an active process pumps Na+ out in the small interstitial space and K+ is pumped into the cell. The basolateral membrane also contains many K+-channel proteins, so K+ will leak back to the interstitial space almost as soon as it has entered the cell. The K+ is absorbed by diffusion - a daily net total of 80 mmol. A Na+-K+-2 Cl- co-transporter located on the basolateral membrane (Fig. 22-15) maintains the Cl- gradient, with an elevated intracellular [Cl-]. This transporter drags Cl- from the interstitial fluid (ISF). The transporter system uses the electrochemical Na+ gradient to transport K+ and Cl- into the cell (Fig. 22-15). The crypt cells hereby can secrete Cl- through the luminal membrane via an electrogenic channel. The Cl- secretion produces a net luminal electronegativity, which drags Na+ across the tight junctions resulting in net secretion (Fig. 22-15). Water (about 2 l daily) is secreted by passive osmosis. A dramatic rise in Cl- and water secretion - caused by gut inflammation with cholera - can lead to secretory diarrhoea. Fluid absorption in the colon is determined by the absorption of NaCl. The Na+ transport involves 1. Electrogenic Na+ transfers via Na+ channels, and 2. Na+-co-exchange as in the small intestine (Fig. 22-14). Both transport processes are driven by the Na+ gradient maintained by the basolateral Na+-K+-pump. (The Na+-solute coupled co-transporter is not present in the human colon). The colonic Na+-K+- pump is more sensitive to aldosterone than that in the small intestine. Aldosterone is a steroid hormone. Steroids bind directly to cytosolic receptors and do not need second messengers. The colonic Na+-K+-pump activity accumulates K+ in the enterocyte, and this gradient drives the K+ secretion across the luminal K+ channel. The Cl- absorption is accomplished by diffusion along a Cl--gradient, and by a luminal Cl--bicarbonate exchanger producing bicarbonate secretion. We have a bicarbonate-chloride-shift just as in the red cells. Since electrolyte absorption exceeds secretion, there is a net water absorption in the healthy colon (1-1.5 l daily and with a colonic salvage capacity of 4 500 ml). Nutrient malabsorption of the small intestine increases the fluid volume delivered to the colon and can provide an osmotic effect in the colon with diarrhoea. Up till 4 600 ml of fluid normally passes the ileocoecal valve without causing diarrhoea. In conditions such as cholera, the excess fluid from the ileum exceeds the colonic salvage, leading to life-threatening diarrhoea. The cholera toxin can enhance the Cl--secretion drastically and cause secretory diarrhoea with large quantities of Cl- and water. In inflammatory diseases of the colon, the colonic salvage capacity is markedly reduced, resulting in colonic diarrhoea. Two-third of the iron content of the body (3-4 g) is stored in the haeme group of haemoglobin. The ability to transport O2 depends on the presence of haeme. Haeme gives the red cell its characteristic red colour. Only haemoglobin with iron in the ferrous state binds O2, whereas the dark red methaemoglobin with the iron in ferric state cannot bind O2. Soluble ferritin forms an intracellular store (25% of total). Essential, but minor amounts of iron, is bound in myoglobin and in the electron-transporting enzymes of the mitochondria in all respiring cells. Haemosiderin is an insoluble degradation product of ferritin that aggregates into cytoplasmic granules. Haemosiderin is a normal microscopic finding in the spleen, bone marrow and the Kupffers cells of the liver. 1. Ascorbate in the food reduces Fe3+ to Fe2+, and forms a soluble complex with iron, thereby effectively promoting the iron absorption. We normally ingest about 20 mg iron daily, and less than 1 mg is absorbed in healthy adults, because iron form insoluble salts and complexes in the gastrointestinal secretions. 2. Iron is transported from the lumen of the upper jejunum, across the mucosa, and into the plasma by an iron-binding protein called gut transferrin. 3. Receptor proteins in the brush border membrane bind the transferrin-iron complex, and the complex is taken up into the cell by receptor-mediated endocytosis (Fig. 22-16). Fig. 22-16: Iron absorption through an enterocyte. 4. There is a free pool of iron in the cytosol. Iron exists in one of two states in the cytosol: The ferrous state (Fe2+) or the ferric state (Fe3+). The Fe2+ ions, after absorption into the mucosal cell, are oxidised to Fe3+ (Fig. 22-16). 5. When intracellular iron is available in excess, it is bound to apoferritin, an ubiquitous iron-binding protein, and stored within the mucosal cells as ferritin. The synthesis of apoferritin is stimulated by iron. This translational mechanism protects against excessive absorption. 6. At the basolateral membrane the Fe3+ are reduced to Fe2+ and passes from the interstitial space to the blood. Here Fe2+ are again oxidised to Fe3+ and binds to plasma transferrin. Cellular iron stores are mobilised by autophagocytosis of enterocyte ferritin, when body stores of iron are deficient. Normally, serum-iron is 12-36 mM, which is about one-third of the total iron-binding capacity in the plasma of adults. This means that one-third of the circulating plasma transferrin is saturated with iron. In iron deficiency the serum-iron is falling, whereas the iron binding capacity increases. The red cell count, haematocrit and the haemoglobin concentration fall in continued deficiency, as does the concentration of iron containing cellular enzymes. Latent (or untreated) iron deficiency anaemia is found in 25-33% of all fertile females. Increase of the total iron content takes place by enhanced intestinal iron absorption or by blood transfusions. Ferritin is further saturated with iron to form Haemosiderin in the liver and elsewhere, when abnormal amounts are ingested over months. Extreme accumulation of excess iron in cells throughout the body (heart, lungs, pancreas, kidneys, glands and skin) finally damages vital organs and is called haemochromatosis. When blood-containing products are ingested, proteolytic enzymes release the haeme groups from the haemoglobin in the intestinal lumen. Haem is absorbed by facilitated transport. Approximately 20% of the haem iron ingested are absorbed. Blood containing products are effective in iron deficiency anaemia. The following is a short description of classical gastrointestinal disorders, such as: 1. Achalasia, 2. Gastro-oesophageal reflux, 3. Gastritis, 4. Peptic ulcer disease, 5. Gastric tumours, 6. Gastrointestinal bleeding, 7. Coeliac disease, 8. Crohns disease and ulcerative colitis, 9. Diarrhoea, 10. Acute abdomen, 11. Colon irritabile, diverticulosis and constipation, 12. Megacolon, 13. Colonic cancer, 14. Dry mouth, and 15. Carbohydrate malabsorption. Achalasia is a disease characterised by lack of peristalsis in oesophagus and relaxation failure of the lower oesophageal sphincter (LOS or american LES) in response to swallowing (Fig. 14-2). Vomiting and weight loss is major symptoms. There is no receptive relaxation, because the myenteric plexus does not work. The aetiology is unknown. There is absence of ganglion cells in the myenteric plexus of the oesophageal wall and the LOS. The peptidergic neurons in the LOS normally secrete VIP (Vasoactive Intestinal Peptide), which relaxes the LOS, but these neurons are lost in achalasia. The food gets stuck because of the lack of peristalsis, the oesophagus dilates and the patient regurgitates. Intermittent dysphagia during meals is typical. Many patients leave the table, provoke vomiting and are relieved. Vomiting is a classical vagal reflex phenomenon relaxing LOS. Fig. 22-17: Oesophageal disorders The diagnosis is confirmed by chest X-ray in particular following a barium swallow, and oesophagoscopy is necessary to exclude malignancy in the region. A pneumatic bag is placed in the LOS opening and pressurised until LOS is sufficiently dilatated. Surgical division of the LOS muscle is performed by laparoscopy. American trypanosomiasis (Chagas´ disease in Latin America) produces achalasia by microbial destruction of the ganglion cells. 2. Gastro-oesophageal reflux disease Gastroesophageal reflux with oesophagitis is caused by incomplete closure of the LOS. Gastric contents with acid reaction then reflux into the oesophagus causing inflammation, erosion and bleeding. This disorder is also called reflux oesophagitis. It results from regurgitation of gastric contents (with HCl and pepsin) into the lower oesophagus causing long lasting damage of its mucosa. The wall becomes hyperaemic, and white patches are seen on the epithelium (leucoplakias). The dysphagia most often presents as heartburn. As dysphagia progress it is likely that an oesophageal stricture is developing. If the squamous epithelium of the lower oesophagus is replaced by columnar epithelium, as a response to long lasting injury, there is an increased risk of transformation of the epithelium into an adenocarcinoma. The most important barrier to the reflux is the LOS. Normally, LOS contracts as soon as the food has passed into the stomach, and the oesophagus is cleared by secondary peristalsis. Gastro-oesophageal reflux disease is usually treated with H2-receptor antagonists, who inhibit the gastric acid production, or with proton pump inhibitors, which inhibit the gastric proton pump and thus effectively reduce gastric acidity. Major complications such as strictures usually need surgery. Gastritis occurs as at least two typical manifestations: Acute, erosive gastritis and chronic, non-erosive gastritis. Focal inflammatory lesions of the mucosa characterise acute gastritis. Sometimes the erosions extend into the deeper layers of the wall (beyond the lamina propria) to form acute ulcers (Fig. 22-18). Acute gastritis is produced by alcohol, drugs (corticosteroids, ASA and NSAIDs) or infections with Helicobacter pylori or virus. After severe stress the gastritis may develop into a life-threatening condition with stress ulcers and haemorrhage. The stress conditions are severe burns, trauma, shock, and sepsis. Chronic gastritis is a long-lasting inflammation of the gastric wall. The superficial layers are infiltrated with lymphocytes and plasma cells. Atrophia develops with loss of both parietal and chief cells. Helicobacter pylori are the chief cause of chronic gastritis in the antrum. The loss of parietal cells leads to achlorhydria (absent HCl production), and to deficiency of intrinsic factor. Autoimmune gastritis is a pangastritis, where autoantibodies to parietal cells can be demonstrated in the blood. Vitamin B12 is not absorbed in the ileum in the absence of intrinsic factor, so the result is pernicious anaemia (Chapter 8). Fig. 22-18: Peptic ulcers extend beyond the lamina propria, whereas erosions are superficial. Peptic ulcer disease is a mucusal ulcer in an acid- producing zone in the distal stomach or the proximal duodenum. The normal stomach produces enough mucus and alkaline juice to protect the gastric and duodenal mucosa against HCl. The mucine molecules swell and form a non-stirred layer covering the mucosa. In duodenum the pancreatic bicarbonate creates a pH of 7.5 at the luminal membrane of the mucosa. Epidemiological occurrence can be explained on the prevalence of Helicobacter pylori infection of the stomach and the colonisation of the upper gastrointestinal tract with this bacteria. Helicobacter pylori infection destroys the protective system, and at the same time provokes excess acid secretion. The patient, whose pain complains typically occur a few hours following a meal or awaken the patient at night, points out Epigastric pains. Bleeding from ulcers can be fatal. Upper gastrointestinal tract bleeding implies a significant loss of blood into the lumen of the foregut. Haematemesis and melaena demonstrate such a bleeding. Haematemesis is defined as vomiting of whole blood or blood clots. Melaena is defined as passage of dark tarry stools (coal-black, shiny, sticky, and foul smelling). Risk factors for peptic ulcer disease are drugs (ASA, NSAIDs and corticoids), hyperparathyroidism (the high Ca2+ level stimulates gastric acid secretion), and gastrin-producing tumours of the pancreas (Zollinger-Ellisons syndrome). Other contributing factors are increased pepsinogen from the chief cells, increased parietal cell mass, reduced somatostatin secretion from the antral D cells, and damage of the mucosa. Acetylsalicylic acid and other non-steroid anti-inflammatory drugs deplete the gastric mucosa for prostaglandins, which leads to mucosal damage. Strong alcoholic beverages also damage the gastric mucosal barrier and stimulate acid secretion. Caffein stimulates gastric acid secretion. Genetic factors must be considered, since persons who do not secrete blood group 0 antigen into the saliva and gastric juice, have an increased risk of developing duodenal ulcers. The diagnosis is confirmed with endoscopy and biopsy or with double-contrast barium technique. The following five therapeutic strategies are used in the treatment of peptic ulcer disease: 1. Eradication of Helicobacter pylori with antibiotics is the treatment of choice for most cases of peptic ulcer disease, since it seems to cure the patient. Clarithromycin is a macrolide that binds to and prevents translocation on Helicobacter pylori- ribosomes, which is an effective basic therapy of peptic ulcers. 2. Inhibition of the gastric proton pump in the luminal membrane of the parietal cells. Omeprazole is a proton pump inhibitor, which relieves symptoms and cure most duodenal ulcers within four weeks - often in combination with antibiotics. Omeprazole and similar antagonists to the gastric proton pump are especially effective in treatment of persistent HCl-secretion caused by the Zollinger-Ellison syndrome. 3. Histamine acts through H2 receptors on the basolateral membrane of the parietal cells. The second messengers for histamine is cAMP. All other cells contain H1 receptors. Accordingly, H2 receptor antagonists (cimetidine, ranitidine, famotidine, and nizatidine) inhibit acid secretion because they fit the H2 receptors specifically. The H2 receptor antagonists prevent histamine from binding to the H2 receptors on the basolateral membrane of the parietal cells. 4. Prostaglandin E1 analogues, such as misoprostol, inhibits gastric acid secretion by unspecific inhibition of the second messenger, cAMP, in the parietal cell and elsewhere. Prostaglandin E1 analogues hereby promote ulcer healing. 5. Surgical management is rarely used unless complications occur. Highly selective vagotomy, in which only the nerve fibres to the parietal cells were cut was previously used, but this is not an alternative to chemical vagotomy (procedure 2., 3., 4.). All treatment procedures, which work by inhibition of gastric acid secretion, have a common drawback. To the extent that gastric acid secretion is reduced there is no inhibition of the gastrin release from the antral G cells. Accordingly, the blood [gastrin] increases, and during treatment of the patients this concentration is constantly increased. The high gastrin level counteracts the expected effect on the acid production. Since gastrin is a trophical hormone for the gastric mucosa, long-term treatment with acid suppression might result in mucosal hypertrophy with a further rise in acid production and in cellular modifications. These complications are probably related to the rather high ulcer recurrence rate of most treatment procedures. Obviously, the only rational strategy is to eliminate the cause of the peptic ulcer disease. The leiomyoma is the most frequent benign gastric tumour. This is a tumour of smooth muscle cells. Leiomyoma are usually discovered at autopsies or by chance, as they do not produce symptoms except when they ulcerate and bleed. Carcinoma of the stomach is frequently located in the antrum and is almost always adenocarcinoma. Risk factors for gastric cancer are Helicobacter pylori colonisation with chronic gastritis, atrophia and metaplasia. Dietary factors include spiced, salted or smoked food (with benzpyren). Nitrosamines are probably carcinogenic in man, and they are produced in food and water with a high nitrate content. One third of the general population have blood group A, but 50% of all patients with gastric cancer belong to blood group A. Enterochromaffin cells of the intestinal wall form carcinoid tumours. The tumour secretes serotonin, bradykinin, histamine, tachykinins and prostaglandins. Somatostatin is an almost universal hormone-inhibitor. A somatostatin analogue, octreotide, inhibits the secretion of many gut hormones including those outlined above. Often the typical signs of carcinoid tumour, facial flushing and diarrhoea are totally alleviated with octreotide treatment. Acute gastrointestinal bleeding occurs in the form of haematemesis or dramatic vomiting of blood. A bleeding peptic ulcer causes most cases. Less frequent is bleeding oesophageal varicose veins, and gastric carcinoma. The danger is bleeding shock, with tachycardia, falling blood pressure and pallor in a cold sweating patient. Urgent and adequate blood transfusion is life saving. Ulcers, infections, tumours, polyps, and varicose veins throughout the gastrointestinal tract cause chronic gastrointestinal bleeding. These patients present with iron deficiency anaemia (Chapter 8). The patients are first examined with gastroscopy, often followed by Colonoscopy or enteroscopy. Gluten-sensitive enteropathy or coeliac disease (sprue) describes a condition where the duodenal and jejunal mucosa is more or less destroyed by hypersensitivity towards gluten (see Chapter 32). 8. Crohns disease and ulcerative colitis These two disorders may be different manifestations of a single disease, non-specific inflammatory bowel disease (see Chapter 32). This term is usually used for an increased stool frequency and implies a larger than normal stool weight (Fig. 22-19). One pathophysiological differentiation of diarrhoea is the following: 1. Zollinger-Ellisons syndrome with tremendous gastric secretion can cause diarrhoea. Fig. 22-19: Diarrhoea of different origin. 2. Bacterial or Secretory diarrhoea is caused by increased Cl - -secretion and reduced Na+ - reabsorption. Enterotoxins from bacteria on the microvillus surface affect the toxin receptors, which increases the cAMP level in the cell. This in turn activates the chloride- channel and inhibits the NaCl reabsorption process. 3. Inflammatory diarrhoea is caused by mucosal destruction with outflow of fluid and blood such as in ulcerative colitis. 4. Osmotic active substances in the gut lumen cause osmotic diarrhoea. These substances are normal nutrients in case of malabsorption, or non-absorbable substances taken for some reason or other. 5. Diarrhoea following ileal resection. Bile acids are normally reabsorbed in the terminal ileum. Following ileal resection the bile acids enter the colon. Bile acids are toxic to the colonic mucosa and stimulate colonic secretion of large volumes, Acute appendicitis is the dominant cause of acute abdomen. Mechanical obstruction of the orifice of the appendix by a faecolith is demonstrated in less than half the operated cases. Secretions dilatate the obstructed appendix, until the mucosa ulcerates and the wall is invaded by intestinal bacteria. In many cases only generalized inflammation is found, and in 10% of all removed appendices, the microscopy is normal. The patient typically experiences periumbilical or diffuse pain, which moves towards the right iliac fossa within hours. The patient is subfebrile and there is nausea and vomiting. The examinator finds a tender right iliac fossa with defence musculaire (guarding), showing local peritonitis. Rectal exploration often reveals tenderness to the right. Perforation of an inflamed appendix can cause several severe complications: Periappendiceal or hepatic abscesses, fistulae, generalized peritonitis, and septicaemia with septic shock. Appendectomy is performed as early as possible by open surgery or by laparoscopy. A history of more than 48 hours of abdominal pain, with a solid mass in the right fossa iliaca indicates disaster. Perforation is most likely present with formation of a periappendiceal abscess. Here, the patient is preferably treated with antibiotics for some days, and appendectomy is delayed (French: a fraud) until the danger of generalized spread to the peritoneal cavity is minimal. Acute peritonitis is frequently caused by perforation and presented as a sudden, severe abdominal pain. High fever develops rapidly with nausea, vomiting and paralytic ileus. As the bacterial infection spreads to affect the peritoneum in general, the condition becomes serious and septic shock may develop. Spontaneous peritonitis with ascites in adults is caused by hepatic, alcoholic cirrhosis with portal hypertension (see Chapter 23). 11. Colon irritabile (irritable bowel), diverticulosis and constipation These are disorders of slow colonic motility. The patient with irritabile bowel syndrome complains of abdominal pain (diffuse or localised to the left iliac fossa), which is relieved by defecation or flatulence. There are often frequent small-volume stools, but the patient feels that the emptying is incomplete. The abdomen is distended. This is a condition with painful spasms causing constipation alternating with mucous diarrhoea. The condition is related to stress and sedentary life style, and is relieved by daily exercise. Fig. 22-20: Frequent colonic disorders Diverticulosis or diverticular disease is a condition with herniation of the mucosa through the muscular layers of the colon, caused by increased intraluminal pressure. The diverticules are recognized following a barium enema, and if they are inflamed the condition is called diverticulitis. Persons with disturbed stool-habits are likely to develop increased intraluminal pressure during defaecation, and they may develop hernias at weak spots in the gut wall. The incidence is high in inactive persons and low in vegetarians or in persons with a high dietary fibre content. Mild clinical cases can be treated with light daily exercise such as walking in a hilly environment. Emergency cases may need surgery. Constipation is frequently caused by a low fibre intake in sedentary persons. They often exhibit irregular defaecation habits, and irrational use of laxatives. Such habits suppress the natural reflexes. The condition is improved by a high-fibre diet or by daily walking. Suppositories may be necessary, but long-term use of laxatives is contraindicated. Megacolon covers several disorders, where the colon is dilatated. Congenital megacolon or Hirschprungs disease is colonic dilatation resulting from congenital absence of ganglion cells in the myenteric plexus at the region, where the colon passes into rectum. Migration of cells from the neural crest is disturbed. The cause is mutation of a gene localised on chromosome 10. The a-ganglionic segment is permanently contracted and stenotic, so the intestinal content is accumulated proximal to stenosis. The markedly distended colon gives rise to the term megacolon. Large amounts of faecal matters accumulate, because peristalsis and mass movements are impossible. The diagnosis is confirmed by a transmural rectal biopsy showing absent ganglion cells. Surgical removal of the segment cures most of the young patients. Fig. 22-21: Hirschprungs disease with a-ganglionosis and megacolon. Acquired Megacolon usually occurs in adults with Parkinsonism, diabetic neuropathy, Chagas disease (Chapter 33) or any other disorder that affect the innervation of the smooth muscles. Colonic cancer is related to slow passage of faecal material with carcinogens through the colon. Carcinogens are chemicals, whose end-products bind to DNA and damage it. Sedentary persons have a high frequency (morbidity) of constipation and a high mortality of colon cancer, but not of rectal cancer. The colon cancer is clearly related to an inactive life style, and regular exercise reduces morbidity and mortality. Prolonged accumulation of faecal content with carcinogens in the colon increases the exposure time of the mucosa and may be of importance. High-fibre diet and daily walking reduce the exposure time. There is a firm correlation between colonic cancer and the activity level of persons in industrial societies. The same is true for groups of persons living on a low fibre diet with a high content of meat and animal fat. Usually the recto-sigmoid area is involved, a location where the faecal content is moved to and fro for varying periods (Fig. 22-22). Patients with chronic gastrointestinal bleeding usually present with iron deficiency anaemia. Measurements for faecal occult blood are easy to perform and of value as a mass population screening for large bowel malignancy. Fig. 22-22: Colon cancer in the ascending colon (polypoid) and in the sigmoid (constricting cancer). – A rectal cancer tumour is shown in the upper rectum. A correlation between rectal cancer and exposure time for carcinogens is not to be expected, because the faecal content passes this part of the tract without delay. A correlation has also been disproved in large population groups. Patients with a rare autoimmune disorder (the Sjögren syndrome) suffer from dry mouth (xerostomia), dry eyes (xerophtalmia) and rheumatoid arthritis. In patients lacking functional salivary glands, xerostomia, infections of the buccal mucosa, and dental caries are prevalent. In most cases of xerostomia the condition is therapy-resistant and unexplained. Some cases are caused by dehydration or by antidepressants. 15. Carbohydrate malabsorption The most common chronic disorder in humans is lactose malabsorption or hypolactasia (lactose-induced diarrhoea or lactose intolerance), which is due to a genetically deficiency of lactase in the brush-border of the duodeno-jejunal enterocytes (see Chapter 31). I. Each of the following statements has True/False options: A. The receptive relaxation response of the stomach decreases Gastroesophageal reflux. B. The intrinsic innervation of the digestive, secretory epithelium responds to parasympathetic input with decreased secretion. C. The sympathetic nerve fibres to the gut act presynaptically to inhibit acetylcholine release in the myenteric ganglia and activate a-receptors. Hereby, sphincter muscles are contracted, blood vessels are constricted, and secretion is inhibited. D. Relaxation of the lower oesophageal sphincter is not caused by increased vagal inhibitory fibre discharge. E. Oesophageal reflex activity is controlled by primary peristalsis that are co-ordinated by a swallowing centre in the solitary tract nucleus, vagal nuclei, and reticular formation. Local distension stimulates the secondary peristalsis. II. Each of the following statements has False/True options: A. Gastrin originates in the antral and duodenal mucosa, where it is released from G-cells. B. Secretin is a hormone that is released from the duodenum in response to HCl. C. Pancreozymin (CCK) contracts the sphincter of Oddi. D. GIP stimulates insulin secretion. E. GRP is involved in vagal gastrin secretion. III. The following five statements have True/False options. A. The major source of cholesterol is food intake. B. A sweat test resulting in a Na+ -concentration above 60 mM in the sweat, is strongly indicative of cystic fibrosis. C. G-cells in the pancreatic islets produce large amounts of a certain hormone, but they are named after their G-protein systems, which amplify a signal, by production of second messengers. D. Glucagon stimulate glycogenolysis, gluconeogenesis, ureagenesis and ketogenesis. E. VIP controls the bloodflow of the gastric mucosa, and GRP releases gastrin from the antral G-cells. IV. Each of the following five statements have False/True options: A: The basic electrical rhythm is an electrical event that always causes contractions in the digestive system. B: The basic electrical rhythm determines the maximal rate of peristaltic contractions. C: Slow waves in the colon cannot result in anti-peristalsis. D: The major role of the human colon is to reabsorb water and electrolytes. E: The only entirely voluntary motor process of the motility patterns in the digestive tract is chewing. V. Each of the following five statements have False/True options: A. Hot and acidic liquids are buffered by saliva in the mouth, and the salivary epidermal growth factor promotes the healing of wounds. B. The parotid secretion is watery and serves to solubilize food, so it can be tasted. C. Salivary buffers maintain the activity of amylase during the first period in the stomach. D. Saliva has bactericide effects due to lysozyme. E. AIDS is transferred via saliva. VI. Each of the following five statements have False/True options: A. Acetylcholine, gastrin and histamine stimulate gastric acid secretion. B. H2 blockers bind to histamine receptors at the basolateral membrane. C. The parietal cells increase their O2 consumption, acid secretion, intracellular [cAMP] and [Ca2+], when stimulated by histamine. D. The H+-K+-ATPase is responsible for gastric acid secretion. E. Gastrin and acetylcholine does not release IP3. A resting male patient, age 54 years, body weights 76 kg, is suspected of Zollinger-Ellison syndrome and examined in the morning after fasting overnight. The throat is sprayed with lignocaine and a gastroscope is introduced into the pharynx under direct vision and passed down the oesophagus into the stomach and duodenum. No ulcers, tumours or bleeding is found. A biopsy of the mucosa shows an overgrowth of parietal cells. A sample of gastric juice is aspirated. Following stimulation by an injection of pentagastrin, gastric juice is aspirated via a nasogastric tube for one hour. The hydrogen ion concentration in the aspirate is 150 mM, and the volume is 350 ml. Due to lung complications the blood gasses of the patient are measured in the morning (PaCO2 40 mmHg, pHa 7.40 , Base Excess zero, actual [bicarbonate] 24 mM) and just after completion of the aspiration (PaCO2 40 mmHg, pHa 7.48, Base Excess 7 mM, actual [Bicarbonate] 30 mM). The next morning blood gases were normalised. 1. Calculate the gastric acid secretion rate of the patient, and compare the result with a normal value of 30 mmol per hour. 2. Describe the acid-base status of the patient just following the aspiration. 3. Explain the normalisation of the acid-base status the following morning. 4. Suggest a better diagnostic tool for the Zollinger-Ellison syndrome. A nervous, smoking male, age 36 years, is admitted to hospital with severe hunger Epigastric pain reduced by eating, acid hiccups, diarrhoea, and steatorrhoea (ie, fatty stools). He has a stressful work, and over the last months he has frequently used drugs containing acetyl salicylic acid for headache, and used whisky on the rocks. Radiological examination of the stomach and duodenum suggests the presence of an ulcer in the duodenal bulb. This is confirmed by endoscopy. Gastric juice is removed by aspiration. The basal rate of HCl secretion is found to be 5 times normal. Histological examination of the gastric mucosa reveals a higher density of parietal cells and gastric glands than normal, but no hyperplasia of antral G cells. The serum [gastrin] of the patient is 10 times higher than normal, and does not increase following a test meal. One dose of the proton pump blocker, Omeprazole, reduces the HCl secretion rate of the patient to normal for 24 hours.
A 35-year old male computer expert visits his general practitioner complaining of exhaustion. For weeks he has suffered from constipation, fatty stools and abdominal pain. He is loosing weight and gets out of breath when he is stair climbing.The patient looks pale and emaciated. Palpation of the abdomen reveals a soft mass in the right iliac fossa. Haematological tests show that the blood haemoglobin is 5.2 mM, the red cell count is (3.1*1012) l-1 and the mean cell volume is 68 fl. Endoscopy with a duodenal biopsy show a normal mucosa with long intact villi. Colonoscopy shows patchy reddening of the mucosa and biopsies show granulomas in the lamina propria. A barium examination reveals narrowing of the terminal ileum. 1. What is the haematological diagnosis? 2. What is wrong with the intestine of the patient? 3. What is the therapy of this condition? 4. Describe the complications of this chronic condition. 5. Describe two disorders which may mimic the condition of this patient. Try to solve the problems before looking up the answers. · Epithelial and glandular cells of the gastrointestinal tract produce important digestive secretions that contain electrolytes, enzymes and hormones. The control of gastrointestinal secretion is effected ny neurons and by hormones. · Saliva is a hypotonic fluid with high bicarbonate and potassium concentrations, and an a-amylase that cleaves a-1-4-glycoside bindings in starch. · Saliva cleans the mouth and pharynx (prevents caries), and ease swallowing. Salivary lysozyme lyses bacterial cell walls. The salivary epidermal growth factor promotes the healing of wounds. · Swallowing is a reflex controlled by brainstem neurons forming a swallowing centre. · The swallowing and vomiting mechanisms are blocked by deep anaesthesia and by injury of the 5.th, 9.th or 10.th cranial nerve. · Gastric motility mixes food with gastric juice and subdivides solids to form a fluid composed of small particles. · Gastric glands and mucosa secrete gastrin (G-cells), HCl (parietal cells), pepsinogen (peptic cells), and mucus (mucous neck cells). Mucus and bicarbonate protect the gastric mucosa from adverse HCl effects. · Segmentation mixes the content of the small intestine. · The migrating motor complex is the “intestinal housekeeper”, which cleanses the gastrointestinal tract. · Vagal, cholinergic preganglionic fibres transfer signals to the gastrin-producing G-cells in the mucosa via non-adrenergic, noncholinergic (NANC) postganglionic neurons. These enteric neurons liberate gastrin-releasing peptide (GRP) to the G-cells. · The ileocoecal sphincter prevents retrograde flow of colonic matter. The sphincter regulates emptying of ileum some five hours after a meal. The emptying of ileum is stimulated by gastrin, possibly via the gastroileal reflex, but a distended colon inhibits the emptying. The ileocoecal sphincter is normally passed by one litre of faecal matters daily. · In the ascending colon, peristalsis is followed by antiperistalsis, which allow time for absorption of water and electrolytes. · Gluten-sensitive enteropathy or coeliac disease describes a condition where the duodenal and jejunal mucosa is more or less destroyed by hypersensitivity to wards gluten. Gluten is found in barley, rye, wheat and oats. · Acetylsalicylic acid and other non-steroid anti-inflammatory drugs deplete the gastric mucosa for prostaglandins, which leads to mucosal damage. Strong alcoholic beverages also damage the gastric mucosal barrier and stimulate acid secretion. Caffein stimulates gastric acid secretion. · Peptic ulcer disease is a mucosal ulcer in an acid-producing zone in the distal stomach or the proximal duodenum. · All treatment procedures, which work by inhibition of gastric acid secretion in peptic ulcer disease, have a common drawback. To the extent that gastric acid secretion is reduced there is no inhibition of the gastrin release from the antral G cells. Accordingly, the blood [gastrin] increases, and during treatment of the patients this concentration is constantly increased. The high gastrin level counteracts the expected effect on the acid production. · Eradication of Helicobacter pylori with antibiotics is the treatment of choice for most cases of peptic ulcer disease, since it seems to cure the patient. Clarithromycin is a macrolide that binds to and prevents translocation on Helicobacter pylori- ribosomes, which is an effective basic therapy of peptic ulcers. · Inhibition of the gastric proton pump in the luminal membrane of the parietal cells. Omeprazole is a proton pump inhibitor, which relieves symptoms and cure most duodenal ulcers within four weeks - often in combination with antibiotics. Omeprazole and similar antagonists to the gastric proton pump are especially effective in treatment of persistent HCl-secretion caused by the Zollinger-Ellison syndrome. · Histamine acts through H2 receptors on the basolateral membrane of the parietal cells. The second messengers for histamine is cAMP. H2 receptor antagonists (cimetidine, ranitidine, famotidine, and nizatidine) inhibit acid secretion because they fit the H2 receptors specifically. The H2 receptor antagonists prevent histamine from binding to the H2 receptors. · Crohns disease is a chronic infection or inflammation of the gut with a particular prevalence for the terminal ileum, but it can be located all the way along the tract. · Ulcerative colitis is always confined to the colon. Ulcerative colitis is a mucosal inflammation with haemorrhage and rectal bleeding. Calver, A., J. Collier and P. Vallance. "Nitric oxide and cardiovascular control." Experimental Physiology 78: 303-326, 1993. Furness, J.B. et al. "Roles of peptides in the enteric nervous system." Trends Neurosci 15:66, 1992.
|
||||||||||||||||||||||||||||||||||||||||
Click here to introduce your comments or contributions