New Human Physiology | Paulev-Zubieta 2nd Edition
Chapter 7: neurological and Psychiatric Diseases
| HOME | PREFACE | TABLE OF CONTENTS | SYMBOLS | SECTION INFO | CONTRIBUTORS | LINKS | CONTACT US |
Highlights
Study_ObjectivesPrinciplesDefinitionsEssentials
PathophysiologyEquationsSelf-AssessmentAnswers
Further Reading
|
|
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
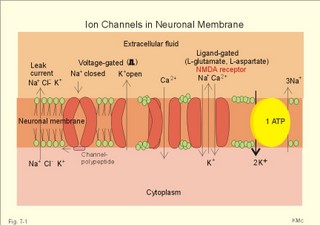
This paragraph deals with 1. Nerve cells, 2. Ion channels, 3. Neurotransmitters, and 4. Signal transduction. The cells of the Central Nervous System (CNS) consist of neuroglia and of neurons. Neuroglial cells outnumber all the neurons in the CNS and they constitute half of the brain volume. Glial cells are known to sheath and protect neurons. Glial cell membranes contain receptors and ion channels. They help control the environment of neurons and thus contribute to the function of neurons. We have three types of neuroglial cells. Microglia cells are small cells scattered throughout the nervous system. Microglia proliferate after injury and move to the site of injury. Here they transform to large phagocytes, which remove the debris. Oligodendrocytes produce myelin sheaths around axons in the CNS just like Schwann cells do in the peripheral nervous system. Astrocytes separate nerve pathways, buffer extracellular [ K+] , and repair nerve injuries. Two classes of proteins span the cell membrane and control ion transfer. The first class is Na+-K+-pumps and other ATP-demanding pumps that actively move ions across the membrane against their electrochemical gradient (Fig. 7-1). The second class is channels or pores through which specific ions can pass. Ions traverse such an open channel along the electrochemical gradient. The small ion permeation through the cell membrane at rest is referred to as leak current (Fig. 7-1). The typical Na+-channel opens promptly in response to depolarisation (voltage-gated opening) and also closes rapidly, although the cell is still depolarised. The channels then remain inactivated for a short period. Opening of Na+-channels increases the flux of Na+ into the neuron, and depolarizes the membrane, so the effect is excitatory. Fig. 7-1: Ion channels in a neuronal membrane, where the Na+-channel is closed and the K+ -channel is open at rest. Closure of K+- or Cl- -channels decreases the flux of K+ out of the neuron or decreases the flux of Cl- into the cell. These events also depolarise the membrane, and again the effect is excitatory. Obviously, closure of Na+-channels or opening of K+- or Cl—channels have an inhibitory effect by hyperpolarisation. Voltage-gated Na+-, K+-, and Ca2+-channels comprise subunits with membrane spanning domains (Fig. 7-1). There is amino acid sequence homology in the transmembrane helices of these channels. The channel protein includes a charged group, which is sensitive to the electric field across the membrane. During depolarisation the gate opens, which changes the whole channel, rendering it much more conductive to specific ions. Each channel continues to open, close and reopen several times during depolarisation. The fast Na+-channels close rapidly and are inactivated during depolarisation due to a channel polypeptide located on the cytosolic side (Fig. 7-1). Opening of Na+-channels requires or results in a rapid change of potential. Partial and slow depolarisation, inactivate a critical fraction of the Na+ -channels. This is called voltage- inactivation. Voltage inactivation of Na+-channels is involved in the accommodation and in the refractory periods. Neurotransmitters are signal molecules used by neurons to communicate with each other and with target cells. Chemical synapses are specialised. The presynaptic terminal contains mechanisms for production and storing of neurotransmitters that are released in response to depolarisation. The postsynaptic membrane carries protein receptors that can detect and identify different neurotransmitters and initiate appropriate responses to stimulation. Finally, there are adequate mechanisms for degradation and reuse of transmitters to ensure rapid onset and offset of arriving signals. Chemical synapses are the sites of action for many drugs. Neurotransmitters can be divided into two groups: Classical rapid acting non-peptide neurotransmitters (Table 7-1) and putative, slowly acting peptides (Table 7-2). During development some process of differentiation determines the type of neurotransmitter that a given neuron will synthesise, store, and release. Thus, a single neuron releases the same neurotransmitter from all its synapses - an assumption, which has been generally accepted for years as Dale’s law. Recent advances indicate that some neurons can release more than one neurotransmitter. Up to 4 neuropeptides have been localised to a single neuron. Two or more transmitters released together are called co-transmitters. One member of each pair of transmitters appears to be a peptide. Perhaps these peptides act by enhancing the message transferred with the rapid neurotransmitters. Classical neurotransmitters are substances such as acetylcholine, noradrenaline, dopamine, GABA (gamma-aminobutyric acid), glycine etc (Table 7-1). Their diffusion pathway is short, and they have no other function than neurotransmission. Catecholamines (dopamine, noradrenaline, and adrenaline) are neurotransmitters both in the sympathetic system and in the CNS. Noradrenaline is the transmitter for most postganglionic sympathetic fibres (some of these fibres use acetylcholine). In the CNS catecholamines are found in several brain nuclei: Dopaminergic neurons are found in the substantia nigra, noradrenergic neurons in locus coeruleus, and serotonergic neurons in the raphe nuclei and in many midbrain structures. Serotonin (5-hydroxytryptamine) is a transmitter in brainstem nuclei (in particular the Median raphe) concerned with wakefulness and behaviour. Adrenaline, noradrenaline, dopamine, and serotonin serve as fast neurotransmitters in the CNS in the same way as the Enzyme- inactivated acetylcholine. The most important excitatory amino acid (EAA)-receptors are the glutamate receptors, the N-methyl-D-aspartate (NMDA)-receptors, and the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-receptors. The glutamate receptor is a typical non-NMDA-receptor. NMDA-activated ion channels are only active, when the membrane is depolarised, and they are specific for Ca2+- Na+- and K+-penetration. AMPA-activated ion channels are specific for Na+-and K+-permeability and depolarise the cell membrane.
Several of the neuropeptides are well-known hormones. They are synthesised in the soma of the neurons and reach the axon terminals by fast axonal transport. The secretion of both gonadotropins (LH and FSH) from the anterior pituitary is controlled by the hypothalamic luteinizing hormone-releasing hormone (LHRH). The thyrotropin-releasing hormone (TRH) and somatostatin from the hypothalamus control the pulsatory secretion of thyrotropin or thyroid stimulating hormone (TSH) from the anterior pituitary. The secretion of growth hormone (GH) and prolactin from the anterior pituitary is controlled by two hypothalamic hormones: GH-inhibiting hormone (GHIH or somatostatin) and GH-releasing hormone (GHRH). Many of these peptides are cut off from a big mother molecule: pro-opio-melanocortin (POMC). Cleavage of POMC in the anterior pituitary lobe releases adrenocorticotropic hormone (ACTH) and b -lipotropin. Cleavage of ACTH in the intermediate pituitary lobe releases melanocyte-stimulating hormone (a -MSH) and corticotropin-like intermediate lobe peptide (CLIP). Cleavage of b -lipotropin releases b -MSH and b -endorphin. Endorphins and enkephalins bind to opiate receptors and are called endogenous opiates. Cleavage of a pre-pro-hormone produced in the hypothalamus releases the two octapeptides, oxytocin and vasopressin together with two neurophysin molecules. The two octapeptides are moved to the neurohypophysis by axoplasmatic transport.
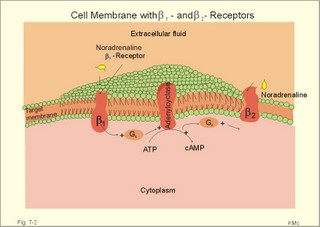
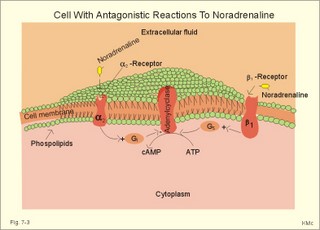
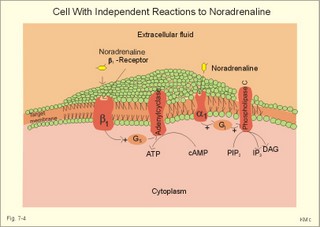
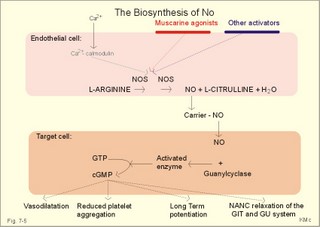
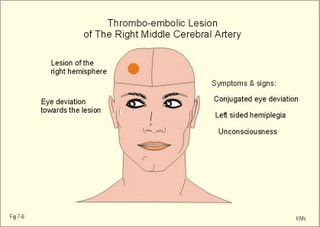
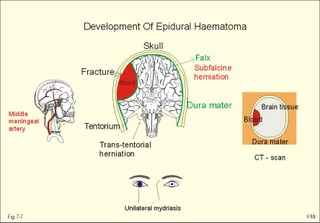
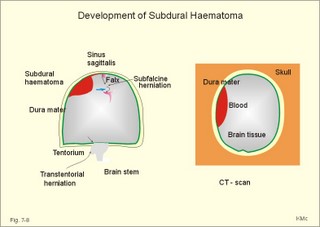
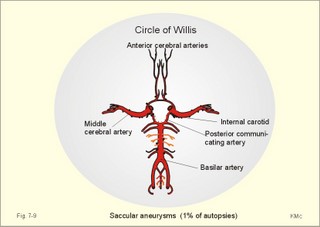
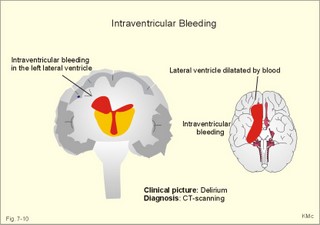
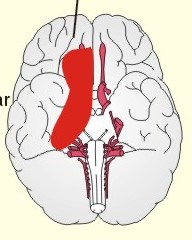
CCK stands for cholecystokinin and VIP for vasoactive intestinal polypeptide. The gut-brain peptides are described in Chapter 22, and the hypothalamo-pituitary peptides in Chapter 26. Signal transduction is a cascade of processes from the receptor-hormone binding to the final cellular response. Many hormones and neurotransmitters raise the concentration of a second messenger in the target cell via guanyl triphosphate (GTP) and act through it. The receptor-hormone complex activates a GTP-binding protein (so-called G-protein) which controls and amplifies the synthesis of the second messenger. Hereby, each hormone molecule can produce many molecules of second messenger such as cAMP or cGMP. Furthermore, each protein kinase unit can phosphorylate many molecules of its substrate, resulting in a great amplification factor. G-proteins function as molecular switches, regulating many cellular processes, such as activation of intracellular enzymes (protein kinase, phosphorylase), activation of membrane enzymes and channels, and activation of gene transcription. G- protein-linked receptors form a family, which has evolved from a common ancestor. Most G-proteins are membrane bound heterotrimers (a, b, g) and exist in an activated state, where it has high affinity for GTP, and an inactive state, where the molecule prefers GDP. Hydrophilic (lipophobic) hormones, such as acetylcholine and many peptides, bind to membrane receptor proteins, and the hormone-receptor binding activates the enzyme phospholipase C via active G-protein. Multiple receptor subtypes can co-exist on a single cell. The b-adrenergic receptors are both stimulated by noradrenaline and both activate a stimulatory G-protein (Gs in Fig. 7-2). Gs activates adenylcyclase, which increases the production of the second messenger cAMP. Fig. 7-2: A single cell with both b1- and b2 -adrenergic receptors. Both receptors activate adenylcyclase through stimulatory G-proteins (Gs). The result is an additive cellular response. A single cell with b1-adrenergic receptors activating adenylcyclase through a stimulating G-protein, and a2 -adrenergic receptors, inhibiting adenylcyclase via an inhibitory G- protein, results in opposite signals when stimulated by noradrenaline (Fig. 7-3). Fig. 7-3: Antagonistic reactions to noradrenaline in a single cell. Noradrenergic stimulation of another single cell with b1-adrenergic receptors activating adenylcyclase through a stimulating G-protein, and a1 -adrenergic receptors which activate phospholipase C leads to production of two phosphorylated derivatives of phosphatidylinositol (PI): PI-phosphate (PIP) and PI-diphosphate (PIP2). Phospholipase C cleaves (PIP2) into inositoltriphosphate (IP3) and diacylglycerol (DAG) (see Fig. 7-4). Fig. 7-4: Independent reactions to the stimulation of two subtypes of adrenergic receptors on a single cell. IP3 is a second messenger that binds to Ca2+-channels in the endoplasmic reticulum (ER), so that Ca2+ is released to the cytosol. DAG and Ca2+ are second messengers that activate protein kinase C, which is involved in the regulation of cellular metabolism, growth and many other processes. Inactive cytosolic protein kinase C is activated by Ca2+, and binds to the inner surface of the membrane, where DAG activates it. Ca2+ and proteinkinase C catalyses the transfer of phosphate from ATP to the effector proteins. Independent reactions are generated by the presence of these two subtypes of adrenergic receptors. Specific receptor-ligand bindings also activate phospholipase A2 via a G-protein. Phospholipase A2 cleaves membrane phospholipids, and releases arachidonic acid (AA) in the cells. AA activates a precursor to platelet activating factor (PAF) termed lyso-PAF. AA is also the precursor for the synthesis of endoperoxides, prostacyclin, thromTableanes (mediates platelet aggregation and vasoconstriction) and leucotrienes. Insulin and related growth factor peptides bind to membrane receptors that are glycoproteins protruding from the membrane. The insulin receptor is typical for this receptor family. Peptide binding to the outer receptor subunit stimulates a protein tyrosine kinase on the inner receptor subunit. This phosphorylates tyrosine residues, both on the receptor itself and on other proteins. The tyrosine kinase activity is essential for signal transduction. Examples of growth factors are: EGF (epidermal growth factor), FGF (fibroblast growth factor), IGF-II (insulin-like growth factor-II), NGF (neural growth factor) and PDGF (platelet-derived growth factor). Protein tyrosine kinase activity is abnormally high in certain types of cancer and cellular modification. This can be caused by growth factors or by a mutation of the tyrosine kinase part of the trans-membraneous receptor. Mutations of one gene localised on chromosome 10 can lead to four different syndromes: Multiple endocrine neoplasia, Hirschprung’s disease, medullary thyroid carcinoma, and Phaeochromocytoma (se Chapter 28). The final step is often phosphorylation or dephosphorylation of a particular key or effector protein. Protein kinases and dephosphorylation accomplish phosphorylation by protein phosphatase. Second messengers (cAMP, cGMP, IP3, DAG, and Ca2+) control the activities of protein kinases such as cAMP-dependent protein kinase A, cGMP-dependent protein kinase, calmodulin-dependent protein kinase, and protein kinase C. Calmodulin binds 4 Ca2+. The phosphorylation level of an enzyme or an ion channel determines and triggers the physiological response. Protein phosphatase reverses the effect of protein phosphorylation. The phosphatase dephosphorylates the key proteins, and thus opposes or stops the physiological response. The free radical gas nitric oxide (NO) is a neuronal messenger in both the central and the peripheral nervous system. The NO gas is membrane permeant, and can bypass normal signal transduction in synapses. Two types of NO synthase (NOS) have been identified: constitutive Ca2+- calmodulin dependent enzyme, and inducible Ca2+ - independent enzyme. Both enzymes are flavoproteins containing bound flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). Both enzymes require the cofactors NADPH and tetrahydrobiopterin (BH4). NOS catalyses the conversion of L-arginine to citrulline and NO in two step when activated by the Ca2+ - calmodulin complex, muscarinic agonists, or other activators (Fig. 7-5). Fig. 7-5: The biosynthesis of NO with cell-cell effects on target cells, such as smooth muscle cells etc. Non-adrenergic non-cholinergic (NANC) relaxation of the gastrointestinal tract (GIT) and the genito-urinary (GU) system is shown. NO diffuses to the target cell, where it activates guanylcyclase resulting in the formation of cGMP (Fig. 7-5). NO is labile. Hence, a carrier for NO has been postulated. The biological effect of NO is mediated by an increase in cGMP levels, and the effects on target cells are shown below (Fig. 7-5). Nitric oxide effects are further developed in Chapters 2, 3, 4, 9, 12, 22, 25 and 30. The NO biosynthetic pathway can be interfered with at several points. Nitrovasodilatators, such as nitro-glycerine, have been used for over a century to treat cardiac cramps or angina pectoris (see Chapter 9). Nitrovasodilatators act by releasing NO and thereby causing coronary vasodilatation. Nitric oxide synthase is inhibited by L-arginine analogues (Fig.7-5). One of the effects of NOS-inhibitors is an increase in blood pressure. NOS-inhibitors are effective in treating endotoxic shock. This is a condition caused by increased NO synthesis by inducible NO synthase, where the sympathetic vasoconstrictors are often ineffectual. The cofactors, like BH4, can also be manipulated, e.g., by anticancer drugs. The first part I concerns neurological disorders of cerebrovascular and brain parenchymal origin (eg, brain trauma, epilepsy, movement disorders, multiple sclerosis, inflammations), and end-up with the differential diagnosis between dementia and delirium - a situation of life-threatening consequences. The second part (II) is confined to psychiatric disorders (eg, neuroses and psychoses). These disorders include parenchymal brain damage, and the main groupings are 1. Stroke and minor stroke, 2.Brain lesions, 3.Epilepsy, 4.Movement disorders, 5.Multiple sclerosis, 6. Inflammations and 7.Intracranial tumours. 8. Dementia contra delirium Thrombo-embolism of the middle cerebral artery is a common cause of stroke (suddenly occurring unconsciousness with hemiplegia). The middle cerebral artery is the artery most often occluded by thrombo-embolism or by atherosclerotic material (Fig. 7-6). Risk factors for stroke are related conditions such as inactivity, obesity, hypertension, smoking, hypercholesterolaemia, hypertriglyceridaemia and oral contraception (see also Chapter 10). Thrombo-embolism causing a stroke usually leads to cerebral infarction. Occlusion of the internal carotid artery or the middle cerebral artery causes infarction of the internal capsule with aphasia (lesion of the dominant hemisphere), contralateral hemiplegia, and areflexic flaccid limbs. The lesion blocks the corticospinal tract as it traverses the internal capsule. Glutamate is released in the ischaemic tissue. The lateral descending system consists of the corticospinal, the corticobulbar and the rubrospinal tracts. Interruption of the lateral descending system to the brainstem and spinal cord causes contralateral paresis, weakness of the finger muscles with loss of fine movements, and loss of the abdominal and cremasteric reflexes. Following the initial spinal shock, a series of release signs are found: Positive sign of Babinski, spasticity (eg, a motor condition dominated by increased tonic and phasic stretch reflexes), foot clonus, and abnormal flexion reflexes. This syndrome is termed the upper motor neuron disease or the pyramidal tract syndrome. The positive sign of Babinski is a slow dorsiflexion of the big toe and fanning of the other toes, when the sole of the foot is stroked laterally from the heel and forward. Fig. 7-6: A stroke patient with thrombo-embolism of the right middle cerebral artery. A pure interruption of the corticospinal tract alone (the medullary pyramid) causes decreased muscle tone and loss of finger movement control, but it does not induce spasticity and flexion reflexes as the lesion of the lateral descending system. Lesions of the medial descending system (ie, the vestibulospinal, reticulospinal, and tectospinal tracts) causes impaired control of the axial muscles, loss of balance during walking, and loss of rightening reflexes. The fine finger movements are normal. A patient with thrombo-embolism in the right hemisphere suffers from left-sided hemiplegia. Head and eyes (conjugated eye deviation) are typically turned toward the lesion. A coma patient, who lacks conjugated eye deviation, is likely to suffer from brainstem injury with severe damage of the reticular activating system. Such a condition has a grave prognosis. Arteriosclerotic brain arteries, micro-aneurysms and larger aneurysms rupture and it bleed into the brain tissue. This primary intracerebral haemorrhage is seen in patients with hypertension. The clinical picture is the same as in thrombo-embolic stroke, although cortical tissue damage with unconsciousness is more common here. Coma is the deepest stage of unconsciousness, where the patient is completely without reactions. Rupture of an arteriosclerotic brain artery or an aneurysm causes bleeding. Bleeding can interrupt the corticospinal tract as it traverses the internal capsule. Such a block of the excitatory pathways to the spinal cord results in severe contralateral paresis, weakness of the finger muscles with loss of fine movements, and loss of superficial reflexes (the abdominal and cremasteric reflexes). This is a typical result of interruption of the lateral descending system, and often termed the upper motor neuron disease or the pyramidal tract syndrome. The capsula interna damage interferes with other cortical efferents to the basal ganglia, the thalamus, and the pons. Therefore, the symptoms and signs are much broader than those after injury of the corticospinal system only are. The stroke patient can slide into deep unconsciousness termed coma. Coma is the deepest stage of unconsciousness. The comatous patient is completely without reactions to even the strongest stimulus. The EEG is dominated by delta waves. When coma proceeds into brain death, the EEG trace shows no electrical activity. Glutamate is released during cerebral ischaemia, such as the ischaemia occurring after a stroke. In animals, N-methyl-D-aspartate-receptor (NMDA) antagonists can prevent ischaemia-induced neurodegeneration. Microembolism or fall in cerebral perfusion may cause a syndrome called transient ischaemic attacks. Small emboli (clots, atherosclerotic material, air or fat) occlude the small arterioles and the brain capillaries. Hypertension can lead to fibromuscular hyperplasia of the walls of parenchymal brain arteries and arterioles. The proliferation reduces the calibre of the arterioles and leads to microinfarction. Multiple microinfarcts impair cognitive functions and lead to dementia. If the retinal artery is temporarily blocked by a microembolus, the patient experiences a sudden transient loss of vision (amaurosis fugax). Temporary bloodflow reduction in the posterior cerebral artery to the medial surface of the temporal lobe causes transient amnesia (memory loss). Transient aphasia is caused by bloodflow reduction to the language comprehension area (Wernicke’s area) of the dominant hemisphere. Transient hypoperfusion of this area causes sensory aphasia (ie, difficulties in understanding written or spoken language, although single words may be recognised). When this occurs in the non-dominant hemisphere, the result is apraxia such as dressing apraxia. The principal causes of head injuries are road traffic accidents and alcohol abuse. Head injury often results in a simple brain concussion, but due to intracranial bleeding the condition sometimes becomes life threatening. The bleeding is located extradurally (epidurally), subdurally or subarachnoid with different degrees of brain parenchymal damage. Computerised tomography (CT) scanning has revolutionised the diagnostic work with head injuries. CT scanning reveals non-invasively the location of blood, skull fractures and cerebral contusion. The location of blood is epidural, subdural, subarachnoid, intraventricular or intracerebral. Head injuries are divided into 2a. Simple concussion and brain contusion, 2b. Epidural haematoma, 2c. Subdural haematoma, 2d.Subarachnoid haemorrhage, and 2e. Intracranial mass lesion. 2a. Simple concussion and brain contusion Simple concussion is defined as a transient loss of consciousness followed by complete recovery. A short period of amnesia is often related to the loss of consciousness. This is a migraine injury, where the duration of the unconsciousness indicates the severity of brain damage. Brain contusion refers to brain damage with prolonged coma, amnesia and focal signs. Later on such patients often suffer from chronic impairment of higher cerebral functions and hemiparesis. Post-traumatic epilepsy is frequently caused by head injury with coma following depressed skull fractures, brain contusion or intracranial haematoma. Actually, depressed skull fracture causes a high incident of post-traumatic epilepsy. Traffic accident victims with severe brain damage may develop the so-called punch-drunk syndrome (dementia with extrapyramidal signs), which typically is found among professional Tableers (Chapter 18) and alcoholics. The middle meningeal artery and its branches are located in the temper-parietal region. Skull fractures in this region or in regions traversing a dural sinus often cause bleeding into the epidural space. Extradural or epidural haematoma is caused by rupture of the middle meningeal artery due to a skull fracture (Fig. 7-7) or by tearing of dural veins to the sigittal sinus. The skull fracture sometimes is accompanied by CSF loss (eg, rhinorrhoea and otorrhoea). Following the head injury with a period of unconsciousness, the patient may appear in a good condition, but suddenly he looses consciousness or develops hemiplegia. Early surgical drainage is lifesaving. A blow to the temporo-parietal region may lead to fracture with transection of one or more branches of the middle meningeal artery (Fig. 7-7). Pulsate bleeding at the high systolic pressure can dissect the dura mater from the calvarium and form an epidural compartment filled with blood. This is a gradual process, because the dura adheres firmly to the bones, and there is usually an asymptomatic interval of 5-6 hours. Since the supratentorial volume is fixed, the expanding haematoma displaces an equal volume from the supratentorial compartment, firstly by reducing the CSF volume, secondly by pressing brain tissue through the orifice as a trans-tentorial and possibly also as a subfalcine hernia (Fig. 7-7). Fig. 7-7: Development of epidural haematoma with herniation and displacement of the falx cerebri. The unilateral pupillary dilatation is caused by oculomotor nerve palsy. As the rising intracranial pressure exceeds the pressure in the large venous sinuses, the veins are compressed and the venous stasis secondly impedes the arterial bloodflow to the brain. The result is cerebral ischaemia (hypoxia and hypercapnia), that occurs even with a high systolic arterial pressure. The high arterial pressure elicits a decline in heart rate via the arterial baroreceptors. Hereby, the ventricular filling is increased, whereby myocardial contractility is increased. The cortical impairment is recognised clinically as confusion and disorientation. The brain tissue is displaced by the growing haematoma, and the tissues of the uncus of the hippocampus are pressed through the tentorial orifice as a trans-tentorial herniation. Hereby, the oculomotor nerve (III. cranial nerve) is pressed against the edge of the tentorium and the resulting nerve palsy is shown as a fixed, dilatated pupil (Fig. 7-7). The clinical picture is that of a simple concussion with a brief period of unconsciousness followed by recovery for some hours. Simple concussion is defined as transient loss of consciousness. The loss is due to traumatic malfunction of neurones in the reticular formation of the brainstem. After 4-8 hours of seemingly recovery, the patient suddenly looses consciousness and develops hemiplegia. The transtentorial herniation is recognised, when the patient is found with an ipsilateral dilatated pupil (Fig. 7-8), but terminally both pupils are fixed and dilated. In the terminal phase tetraplegia develops. The herniated brain tissue also compresses and displaces the brain stem (midbrain, pons and medulla) resulting in venous stagnation of blood and ischaemia. Impaired function of the neurones in the reticular formation and the cardio-respiratory control centres leads to unconsciousness and cardio-respiratory failure. Lack of oxygen for even a short period results in neuronal damage and necrosis, which is irreversible. An epidural haematoma must be recognised and evacuated as soon as possible. Otherwise the bleeding progress until death ensues. Subdural haematoma is an accumulation of blood in the subdural space caused by venous bleeding. The cause is head injury with latency between the event and the symptoms (headache, confusion, stupor, coma, delirium, hemiparesis, epilepsy etc). CT scanning confirms the diagnosis. The latency is sometimes so short, that the clinical picture resembles that of extradural haematoma. The arachnoid is bound to the cerebral hemispheres, but unattached to the dura mater. Veins from the cerebral hemispheres cross the subarachnoid space, penetrate the arachnoid and the dura, and finally enter the dural sinuses. Injuries applied to the frontal or occipital regions initiate shock waves through the liquid brain tissue, whereby the cortical veins are cleaved just before the blood has reached the saggital sinus. Fig. 7-8: Development of subdural haematoma with transtentorial and subfalcine herniation. In this way blood accumulate in the subdural space (Fig. 7-8). The bleeding may be from only one vein, and the development may be slow. Latency between the time for injury and the occurrence of the first symptom can be weeks or months. Headache and confusion are unspecific indications in the elderly. Cognitive functions are often impaired by bilateral subdural haematoma. Manifest dementia is sometimes misinterpreted as senility. CT, MRI or arteriography confirms the diagnosis. Surgical drainage is performed. New bleeding may develop acutely with terminal transtentorial herniation. Some types of subdural haematoma resolve spontaneously. Subarachnoid haemorrhage is a spontaneous arterial bleeding into the subarachnoid space. The clinical picture can be that of delirium. Diagnosis is confirmed with CT scanning, and neurosurgical closure of the aneurysm is sometimes possible. Bleeding into the subarachnoid space is most often spontaneous rather than traumatic. The circle of Willis and adjacent vessels is the most frequent site for saccular or berry aneurysms (Fig. 7-9). Fig. 7-9: The circle of Willis with saccular aneurysms (black). The aneurysms rupture spontaneously, often at rest and the patient experience a sudden, devastating headache followed by loss of consciousness. The neck is stiff and the back is stiff as well. Subarachnoid or intraventricular blood is clearly demonstrated by CT scanning, and in such cases lumbar puncture is unnecessary (Fig. 7-10). Fig. 7-10: Subarachnoid and intraventricular bleeding in the left lateral ventricle (left). The blood-filled lateral ventricle is also projected to the base of the brain (right). Previously, the diagnosis was confirmed by the presence of blood in the CSF. Today, the lumbar puncture is often avoided, as a spinal tap causes a sudden pressure differential between the supra- and infra-tentorial compartments. This may elicit transtentorial herniation with brainstem compression and death. Angiography is performed on patients fit for neurosurgical closure of the bleeding site. located supratentorially (above the tentorium cerebelli) can compress the brain towards the tentorium as to block the upward flow of CSF and thus its absorption. Such mass lesions are brain tumours, encephalitis, meningitis, haemorrhages, aneurysms, brain abscesses, and the effect is similar to the effect of brain contusion just analysed. Hereby, the CSF-pressure below the tentorium cerebelli increases. A rise in CSF-pressure below the tentorium results in papiloedema, because it creates a high pressure inside the optic nerve sheath and thus pushes fluid into the optic disc or papilla. Ophtalmoscopy reveals blurring of the edges of the papilla and dilated retinal veins without the normal pulsation. The papilla looks like the top of a champignon. Lesions of the vessels result in visible, retinal haemorrhages. Continuous intracranial pressure monitoring is important during treatment of comatous patients with severe head trauma. Intracranial mass lesions are almost always surrounded by cerebral oedema. Mass lesions that include the cerebral cortex often lead to epilepsy. Cerebral oedema is caused by increased pressure in the brain capillaries or by lesions of their walls. A rise in cerebral arterial pressure above the upper limit for autoregulation (ie, almost constant bloodflow despite rising driving pressure) results in brain oedema. Brain oedema compresses intracranial vessels, whereby brain bloodflow is reduced and brain ischaemia develops. This is the start of a vicious cycle, because the hypoxia increases the capillary permeability and dilates also the arterioles. Hereby, the brain oedema develops further. Hypoxia also blocks the Na+-K+-pump, whereby the brain cells swell (eg, intracellular overhydration). Intravenous infusion of a concentrated osmotic solution such as mannitol drags oedema fluid from the brain tissue, and benefit the patient. A patient in coma, suspected of increased intracranial pressure, can be treated with 1- 2 g mannitol per kg iv., while further procedures are carried out. Intubation and hyperventilation should also be instituted to any comatous patient. Reduction of PaCO2 to 25 mmHg (3.3 kPa) will rapidly reduce intracranial pressure by decreasing cerebral bloodflow and blood volume. Brain stem compression occurs when intracranial mass lesions above the tentorium damage the ascending reticular activating system (RAS). The high pressure pushes the basal parts of the temporal lobes through the incissura tentorii, and the cerebellar tonsils through the foramen magnum. Brain tissue incarceration with brain stem compression is a serious cause of coma, which must be diagnosed and treated immediately. Suspicion of increased intracranial pressure is contraindication of lumbar puncture, because it may cause brain stem compression by transtentorial herniation. Accordingly, ophtalmoscopy with the exclusion of papillary oedema is necessary before any lumbar puncture. Epileptic seizures are partial or general. Epilepsy is an abnormal paroxystic discharge from cerebral neurones resulting in a condition with clinical consequences. The normal EEG waves are due to synaptic potentials by groups of neurons including pyramidal cells. An epileptic seizure is characterised by high voltage-high frequency discharge (100-200 µV) from large groups of neurons or from the entire cortex. Partial or focal seizures can be caused by an epileptic focus anywhere in the cortex. The causes of focal seizures are acquired lesions such as cysts, tumours, scar tissue, infections, ischaemic lesions. The epileptic discharge causes involuntary muscular contractions on the contralateral side. Foci in the somatosensory cortex produce sensory hallucinations called an epileptic aura. These hallucinations precede the epileptic seizure. The aura varies and is particular for a certain patient. Epileptic foci in the visual cortex cause visual auras, while epileptic foci in the vestibular cortex produce an aura-feeling of spinning. Psychomotor epilepsy originates in the limbic system and causes emotional hallucinations and muscle contractions. Focal seizures are characterised by high epileptic spikes in the EEG. Motor seizures originate in the motor cortex of the opposite side, and they follow a specific pattern in each patient. They are called Jacksonian seizures and often precede the generalised types. Generalised epileptic seizures involve most of the brain and imply loss of consciousness. Generalised absence (petit mal) is a transient loss of consciousness. These short attacks are recognised by spike-doom waves in the EEG. Primary generalised tonic-clinic seizure (grand mal) is characterised by an extreme and widely distributed electrical activity, with tonic-clinic convulsions of the entire body. Presumably, a basic neuronal circuit activates the cortex of both hemispheres in generalised seizures. The hyperactive nerve cells release K+ and glutamate during a seizure. Small children with high fever (purexia) often react with generalised epileptic seizures called febrile convulsions. Diabetics in hypoglycaemia (ie, a blood glucose concentration below 3 mM) may develop generalised convulsions. Epileptogenesis. The genesis and spread of epileptic discharges are poorly understood. The increased cortical excitability, with high voltage-high frequency discharge over the entire cortex, is not explained. Several mechanisms are probably involved:
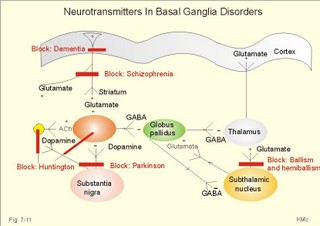
During seizures, the extracellular [ K+] increases substantially, so the resting membrane potential is reduced. This makes the neurons more excitable and promotes the spread of the discharge. Fortunately, phenytoin blocks better at high K+-concentrations around the neurons. Adenosine inhibits the initiation of seizures in experimental animals. Carbamazepine promotes the adenosine-inhibition and thus blocks or reduces epileptogenesis. Emergency therapy of a seizure is to keep the airways patent, apply diazepam suppositories to patients with prolonged seizures, and intravenous glucose in case of hypoglycaemia. The patient must be protected from harming himself during the few minutes of generalised cramps. Long-term therapy of primary generalised tonic-clonic epilepsy (grand mal) and partial epilepsy is frequently made by use of carbamazepine or phenytoin. Generalised absence (petit mal) is frequently treated with sodium valproate. Status epilepticus is life threatening due to cardio-pulmonary insufficiency and must be treated immediately with cardio-pulmonary support and diazepam intravenously. Disorders of the neurotransmission in the extrapyramidal system results in movement disorders of two types. Loss of movement with increase in muscular tone is termed akinetic-rigid syndromes, whereas disorders with involuntary movements are called dyskinesias. 4a. Parkinson´s idiopathic disease or shaking palsy is characterised by tremor at rest, rigidity, akinesia or bradykinesia and postural changes. Parkinson‘s disease is characterised by well-preserved cholinergic activity, but a reduction of the dopamine content in putamen and substantia nigra, of noradrenaline and 5-hydroxytryptamine in putamen, and of the GABA-synthesising enzyme glutamic acid decarTableylase in substantia nigra and in the cerebral cortex. The main pathological mechanism is degeneration of dopaminergic neurons in substantia nigra. The consequence is a severe lack of dopamine in the striatum. The lack of dopamine in substantia nigra hyperactivates the GABA pathways to the thalamus, which activates the motor cortex neurons. This increases the discharge of alpha-motor neurons in the spinal cord resulting in plastic rigidity, akinesia and dyskinesia. L-DOPA is a precursor of dopamine that is capable of crossing the blood-brain barrier. Administration of this drug, which is transformed to dopamine in the brain, relieves much of the rigidity and akinesia by inhibiting the striatum. Transplantation of dopaminergic neurons into the striatum has been explored. Increased dopamine activity relieves rigidity. Too much dopamine causes chorea (see below). Increased acetylcholine activity or reduced dopamine activity causes rigidity and bradykinesia in healthy persons. This is why reserpine, which depletes neurons for dopamine, and butypherones, which block the secretion from dopaminergic neurons, all causes so-called drug-induced Parkinsonism. Drug-induced Parkinsonism is a common side effect in patients treated neuroleptic drugs or in patients given metochlopramide. Akathisia refers to a condition with restlessness and uncontrolled repetitive movements in patients with drug- induced Parkinsonism. Drug-induced Parkinsonism is refractory to usual drug therapy. The patients respond immediately upon seponation of the inducing drug. The tremor is often pill rolling between the fingers and thumbs, and not necessarily bilateral. The stiffness or rigidity is called lead-pipe or plastic rigidity, because the high muscle tone is equal throughout the range of passive movements and the same by flexion and extension. Sometimes the resistance to passive movements is jerky, so-called cog-wheel-movements. Akinesia (hypokinesia, bradykinesia) means inability to initiate normal movements. The face is mask-like with rare blinking and monotonous dysarthria. Postural changes include a forward posture with short step gait and no arm swinging. The patient easily loose balances and falls stiffly to the ground. Fig. 7-11: Neurotransmitters in the basal ganglia. ACh stands for acetylcholine. Dementia or cognitive disturbances are present in some patients. Dementia is ageing of the brain with a resulting loss of mental powers. A balance between the effects of dopamine and glutamate is a necessity for the normal functioning striatum. Patients are treated with an amino acid precursor of dopamine: L-dihydroxyphenylalanine (L-DOPA). This molecule can cross the intestinal-blood barrier and the blood- brain barrier easily. Hereby, L-DOPA reach the inside of neurons (carrier-mediated transport). L-DOPA is normally synthesised in dopaminergic neurons from dietary L-tyrosine. Exogenous L-DOPA is methylated by catechol-O-methyl transferase (COMT) to 3-O-methyl DOPA, or it is decarTableylated by decarTableylase to dopamine. Finally, dopamine is catabolised to homovanillic acid by COMT and monoamine oxidase (MAO). Most of the exogenous L-DOPA is lost by decarTableylation in patients where carbi-DOPA is not administered concomitantly. Carbi-DOPA inhibits the decarTableylase and reduces the side effects of L-DOPA. When Parkinson patients are treated with too much of the dopamine precursor L-DOPA, they may develop hallucinations, fear and paranoid delusions (schizophrenia-symptoms), because excess dopamine causes schizophrenia or schizophrenic symptoms and signs. Chloropromazine and haloperidol decrease the dopaminergic effects and are called anti-schizophrenics. These drugs block both the D1 and the D2 dopamine receptors, so they reduce the dopaminergic effects, but also cause extrapyramidal side effects on the top of cholinergic and adrenergic blockade. Amantidin increases the dopamine release from nerve terminals in the striatum, so this drug is effective mainly in the early phases of Parkinson´s disease, before all dopaminergic neurons are degenerated (Fig. 7-11). Anticholinergic drugs, which block muscarinic, cholinergic receptors, are still in use to treat early Parkinson symptoms such as tremor. The side effects are dry mouth, bladder weakness, constipation, and confusion and memory loss. Parkinson patients develop DOPA resistance following years of dopaminergic medication. The use of antagonists for the glutamate receptor in Parkinson patients decreases the activity of the glutamate pathway to the cortex, and reverses the akinesia and rigidity. 4b.Wilsons disease (hepatolenticular degeneration) is an autosomal recessive anomaly of the copper metabolism. The abnormal gene is located on chromosome 13. Normally, copper is absorbed from the gastrointestinal tract and in the liver it is incorporated into caeruloplasmin. Normally, copper is excreted into the bile. Wilson-children have low serum caeruloplasmin and serum copper. They fail to excrete copper. Copper is accumulated in the basal ganglia of the brain, the liver (liver cirrhosis), and the cornea/lens. Wilson-children show an akinetic-rigid syndrome with liver cirrhosis, haemolysis, anaemia and visual disturbances. Early diagnosis with long-term treatment (penicillamine) has improved the prognosis. 4c. Dyskinesias (Chorea and Myoclonus) Chorea is a type of involitional hyperkinesia, with jerky movements of the limbs. The movements look almost like voluntary, purposeful movements. A special type of chorea is athetosis (aethymology: "not fixed"), which refers to slow, twisting involuntary movements of the fingers. Athetosis is seen in children following brain damage with bilateral damage of the nucleus subthalamicus. St. Vitus dance or Sydenhams chorea minor is a complication following rheumatic fever with encephalitis. The movements are usually unilateral. Recovery typically occurs spontaneously. Hemiballismus or hemichorea describes violent, throwing movements of one arm or one side of the body - as if the patient tried to throw a ball. The cause is partial lesion of the contralateral nucleus subthalamicus - often due to thrombosis. Ballism means flailing movements of the limbs. Huntington’s chorea is an autosomal dominant genetic defect on chromosome 4. Its characteristic disorders are hypotonia, dementia and involuntary hyperkinesia. Huntington’s chorea is due to a defect in GABAergic and acetylcholinergic interneurons of the striatum and the cerebral cortex. The two transmitters are normally synthesised by the enzymes, glutamic acid decarTableylase and acetylcholine transferase, but their concentrations in the interneurons is markedly reduced. The diagnosis is confirmed with genetic testing. The prognosis is bad with rapid mental deterioration in particular in young patients. The dementia of the Huntington’s chorea is caused - as for most types of dementia - by cortical degeneration. GABA receptors are usually inhibitory. When GABA no longer inhibits the globus pallidus from the striatum, this leads to a stronger inhibition of the thalamus, which is probably the cause of the involitional choreiform movements. Chorea is opposed to rigidity. Low doses of a dopamine agonist may reduce the choreiform movements of patients with Huntington’s disease. Myoclonus refers to brief contractions or jerks of one or more muscles. Myoclonus is often called nocturnal myoclonus, because it occurs at night. Generalised myoclonus resembles epileptic seizures, and is related to epilepsy following brain damage by hypoxia. Myoclonus is related to drug toxicity or metabolic toxicity from renal or hepatic insufficiency. Tics are repeated twitching of facial or neck muscles. Tics are also called mimic or focal myoclonus. The tics may begin in childhood for unknown reasons. Tics are extremely resistant to any therapy. Tremor can be caused by hyperthyroidism and by Parkinsonism, but it is also a typical side effect of alcohol, narcotics and drug abuse. Some cases of essential tremor can be reduced by beta-blockers. Multiple or disseminated sclerosis refers to a common neurological disease caused by inefficient myelin production in the oligodendroglia. The cause is unknown, but the acquired defect in the oligodendroglia cells results in demyelinized areas or plaques in the CNS. The prevalence increases progressively with the distance from the equator, and the patients have an abnormal immune response with large concentrations of antibodies to virus infections. The demyelinized plaques are mainly localised to the brainstem, cerebellum, periventricular region and optic nerves. Motor neurons of the spinal cord and peripheral nerves are rarely affected by demyelinisation. Blurring of vision in one eye is usual with disc swelling of the optic nerve at ophtalmoscopy. There is also diplopia, vertigo, nystagmus, and dysphagia, when the brainstem is affected. Later paraparesis and tetraparesis develops. Death ensues by lung infections or uraemia. Magnetic resonance imaging (MRI) with scanning of the brain and CNS can visualise demyelinized plaques in the periventricular white matter or elsewhere. MRI is an expensive technique, where protons are activated with radiofrequency waves to create images. In the CNS, the white and the grey matter are distinguished. This is a disabling disorder for which there is no cure. Interferon has been tried with some effect on the lesions visualised with MRI. Palliative treatment necessitates teamwork. Meningitis refers to inflammation of the meninges. Clinically, the meningitis syndrome is characteristic. The meningitis syndrome is a patient with high fever, headache, photophobia and vomiting. The patient - often a child - is placid and inactive, consciousness may be impaired and neck stiffness develops. Bacteria, viruses, fungi, chemicals or drugs cause meningitis, or unusual organisms in immuno- compromised patients. Immediate administration of intravenous benzylpenicillin is life saving in cases of acute meningococcal or other bacterial meningitis together with urgent investigations. Encephalitis is inflammation of the brain tissue caused by the same organisms as meningitis. Herpes simplex encephalitis is treated with acyclovir intravenously. Acyclovir inhibits DNA synthesis and thus the proliferation of the virus. Japanese B encephalitis is avoided by vaccination of travellers to the Far East. Other causes to acute viral encephalitis are Coxsackie virus, Echo virus and mumps virus. AIDS in the CNS is caused by the HIV itself or the CNS disease is caused by other infectious agents - in particular fungi, TB, or Escherichia coli which damage brain cells. The clinical picture is meningitis, myelitis or encephalitis. Neural syphilis may occur as tabes dorsalis. Tabes dorsalis is caused by demyelinisation of the dorsal roots of the spinal cord. Lancinating pains, ataxia, loss of reflexes, muscle wasting, neuropathic joints, Argyll Robertson’s light-stiff pupils and optic atrophy. Tertiary syphilis can be avoided if the primary syphilis infection is treated correct. Usually, injection of 1 g i.m. Benzylpenicillin for 2 weeks is enough. Rubella encephalitis caused by rubella virus may progress following some years, because of antibody production against rubella viral antigen. Creutzfeld-Jacobs Disease (CJD) and KURU (among cannibals in Papua, New Guinea) are known from the spongioform encephalopathy seen at autopsy - the brain looks like an Emmenthaler cheese. The pathology is similar to that of bovine spongiform encephalopathy of cattle and sheep ("SCRAPIE"). CJD is inherited of transmitted to man with a prion. The prion is an abnormal neuronal membrane protein, which can mutate like a virus. The prion is resistant to usual sterilisation procedures, and the incubation period is not always for years. Prions are transferred when eating neural tissue from sick cows or sheep and in other ways. The first signs in humans are various neurological insults such as sudden blindness, difficulties in gait and balance, memory and concentration disturbances, and slowly progressing dementia until a rapid death. – The hereditary form of CJD is caused by mutation of the human gene (PRNP) for the neuronal membrane protein. Today, KURU is history. The high incidence in New Guinea 30 years ago was due to cannibalistic rituals. Gadjusek showed that KURU was infectious, and received the Nobel Prize in 1976. Most intracranial tumours are primary tumours (75%) and approximately one-quarter are secondary (metastases). The primary malignant tumours are gliomas (eg. astrocytomas and oligodendrogliomas), and the primary benignant tumours are meningeomas and neurofibromas. The metastases originate from primary tumours in the breasts, bronchi, kidneys, prostate, stomach, and thyroid etc. Magnetic resonance imaging (MRI) is a scanning technique, where protons in a strong magnetic field are bombarded with radiofrequency waves in order to produce images. MRI scanning can picture brain tumours, multiple sclerosis lesions, and syringomylia among others. MRI scanning can even separate white from grey matter. MRI scanning is replacing myelography, because it can visualise spinal cord compression, spinal cord tumours and other malformations. Gliomas originate in the neuroglia. Astrocytomas are gliomas originating from astrocytes. Astrocytomas are usually located in the cerebrum in adults, and in the cerebellum in children Oligodendrogliomas originate from the oligodendroglia and grow slowly in the cerebral tissue Meningeomas originate from the arachnoid matter usually along the venous sinuses above the tentorium. They are benign and grow slowly. Neurofibromas arise from Schwann cells usually around the 8th cranial nerve (acoustic Schwannomas). Symptoms and signs of brain tumours are treated already in 2e. Intracranial mass lesions. Dementia (senility or ageing of the brain) disturbs almost all cognitive brain functions, whereby the personality of the patient is completely changed (cognitive functions as calculation, comprehension, judgement, language, learning ability, memory, orientation, and thinking). Dementia develops slowly and has no diurnal variation. Cortical atrophy is found by using CT or MRI scanning of the brain. The clinical differential diagnosis to delirium and depression is sometimes difficult to establish, but it is consequential to the delirious patient, if the diagnosis is misinterpreted as dementia (Table 7-3). Dementia is an exclusion diagnosis.
is a possible cholinergic system disease. Altzheimers disease is a primary (ie, unknown aetiology) cortical brain atrophy. Lack of the acetylcholine-producing enzyme choline acetyltransferase, and of acetylcholine has been demonstrated by neurochemical studies. Alzheimer’s disease is a form of presenile dementia or premature ageing of the brain (ie. occurring before the age of 70). The disease is rapidly progressing to complete loss of mental powers, in particular loss of memory and normal emotional behaviour. CT scan shows cortical atrophy and excludes brain tumours. At autopsy argentophilic plaques filled with amyloid protein A4 are found in the hippocampus, basal ganglia, thalamus and the cortex. The gene defect causing familial Alzheimer disease is located on chromosome 21, close to the pro-A4 gene. The cholesterol transport to the tissues is also affected. Alzheimer’s disease is probably caused by neuronal degeneration in the nucleus basalis close to the globus pallidus, and possibly also to lack of somatostatin and substance P in deep brain centres. Normally, cholinergic axons from the nucleus basalis project to the cortex, and their functions relate to memory and to the limbic system functions. There is no specific treatment of dementia. Anxiety and depression is treated symptomatically (Table 7-3). Delirium is an acute impairment of consciousness also called toxic confusion. Sense impressions are misinterpreted, the mind and memory work incoherently, and the patient is frightened and suspicious because of hallucinations. Relatives often mention senility, but they may also inform about an acute start, so delirium is recognisable. Besides being acutely developing, delirium is also worst at night with visual hallucinations and incoherent speech and perseveration. In contrast, the dementia patient is conscious, cannot find the right words and the development has been slow. The basis for the delirious pattern is organic brain disease caused by intoxication (eg. alcohol, drugs, poisons), brain damage by infections, lesions, subarachnoidal haemorrhage or tumours, systemic infections (malaria, septicaemia, TB), and metabolic brain damage (eg, hepatic or renal failure, hypoxia, vitamin B2, B6, B12 deficiency). The treatment of delirium concentrates firstly on the underlying disease (including electrolyte disorders, ischaemia etc), and secondly on the symptomatic aspect. The international Classification of Disease and Related Health Problems (ICD 10, WHO) is used. The most serious disorders are psychoses, and the most common disorders are neuroses. The description concentrates on the two classical psychoses schizophrenia and manic-depressive psychosis. The following personality disorders (neuroses) are described: Phobic anxiety neurosis, obsessive-compulsive disorders, dissociative-conversion disorders (hysteria), and eating disorders (anorexia nervosa and bulimina nervosa). The pathophysiology of affect and stress is also considered. means splitting of the mind or disconnection of psychic functions (emotional and cognitive). Schizophrenia is a psychosis with hallucinations, dissociation of ideas, intense fear, and paranoid delusions (paranoia). Schizophrenia is possibly caused by hypersecretion of dopamine or by blockage of the glutamate producing neurons from the cortex to the striatum. The balance between these two neurotransmitters in the striatum is seriously disturbed. The clinical syndromes covered by this term include - according to WHO - auditory hallucinations (eg, hearing voices), thought withdrawal with abnormal posture, delusional perceptions with paranoia and external control of emotions with persecution from the outside. The patients’ feel that their thoughts and emotions are broad casted and they not only hear voices commenting their lives, but also their own thoughts are spoken aloud. The cause is sometimes clarified as a biochemical brain damage with hypersecretion of dopamine from neurons in the mesolimbic dopaminergic system close to substantia nigra or by blockade of the glutamate-producing neurons from the cortex to the striatum. An imbalance between the effects of dopamine and glutamate spoils the normal function of the striatum. A special gene located in chromosome 5 increases the risk of schizophrenia. Dopamine agonists such as amphetamine, and other psychotic drugs (LSD, mescaline, and ecstasy) can cause schizophrenic psychosis. The genetic involvement is demonstrated by a 50% risk for the monozygotic twin of an affected person. There is a 40% risk for two affected parents for having a schizophrenic child. The gene is probably located on chromosome 5. Some schizophrenics have limbic dysfunction of the left hemisphere. Schizophrenia begins in young adults of both sexes, and may be more than one entity. Schizophrenics are frequently vulnerable to highly expressed emotions. Chronic schizophrenics are characterised by lack of drive, underactivity, social withdrawal, and emotional emptiness. Catatonia (stupor, stereotypes, and automatic obedience) was previously seen in many institutional patients, and may still be seen among understimulated patients. Dopamine blockers - blocking D1 and D2 dopamine receptors - are the drugs of choice in acute schizophrenia. These drugs belong to the phenothiazine family (chlorpromazine, trifluoroperazine and promazine). Side effects are unavoidable as the drugs block both D1 and D2 receptors, as well as adrenergic and cholinergic receptors. The side effects are extrapyramidal (acute dystonia, Parkinsonism, akathisia and tardive dyskinesia), autonomic (hypotension and ejaculation failure), and anticholinergic symptoms (dry mouth, urine retention, constipation and blurred vision). covers severe abnormalities of mood. Mood ranges from severe depressive psychosis over moderate and minor depression, sadness, normal mood, happiness, euphoria, hypomania, and severe mania. The diagnosis manic-depressive psychosis describes patients with periodic attacks of mania or depression, separated by periods of normal behaviour. The diagnosis also includes patients with depressive periods alone, or with only manic periods. Endogenous depression is characterised by early morning waking with unresponsive sadness, guilt feeling, suicidal feelings, and lack of a precipitating factor. Severe depression disturbs mood, talk and initiative. One type of mental depression is related to reduced formation of noradrenaline in the locus coeruleus, and of serotonin in the midline raphe nuclei of the brainstem, which seriously damage the limbic system. - Other types of depression are called exogenous or reactive depressions, because they are considered to be due to exogenous or environmental factors. Medical drugs that inhibit the production of noradrenaline and serotonin often cause depression. Hypomania is mild mania with euphoria, overactivity and disinhibition. The genetic aetiology of manic-depressive psychosis is confirmed by the concordance of two thirds of monozygotic twins, and by the fact that more than 20 % of dizygotic twins are concordant. There is a clear overweight of females. Winter depression from autumn to spring is frequent in areas with lack of light in the winter months. Up to 20% of the population north of the polar circle suffers from winter depression. Light therapy several hours daily are so effective that overdoses may release manic phases. Monoamine-neurotransmitters are depleted in depression but increased in manic phases. Stressful social life events (marriage, divorce, moving house, loss of job, vacation, etc.) often precipitate depression. Selective serotonin reuptake inhibitors (SSRI) are often preferred in treatment of depressive states, because of rapid effect and lower rate of serious side-effects including addiction. These substances inhibit serotonin reuptake within the synaptic cleft, and are named "happiness pills" in the media. Happiness pills do not exist. Depression was previously treated with monoamine oxidase inhibitors (MAO-inhibitors). They inhibit the enzyme monoamine oxidase A&B and thus the breakdown of monoamines. Hereby, adrenaline, dopamine and 5-hydroxytryptamine are accumulated in the brain. Tricyclic antidepressants block reuptake of monoamines and are likewise effective in the treatment of depressive patients. Electroconvulsive therapy (ECT) is a physical treatment with rapid effect, often used for cases with suicidal or other deep depressions. Therapeutics (such as lithium compounds) that inhibits the action of noradrenaline or serotonin is effective prophylactic agents against manic phases. In this model mania is caused by overproduction of monoamines, and depression by reduced formation of monoamines in the brain nuclei mentioned above. Actually, lithium carbonate is used in the prophylactics of manic phases. A plasma-lithium concentration of 0.5-1 mM is necessary to obtain an acceptable result. Psychoses are treated with antipsychotics often supplemented with benzodiazepines. The typical antipsychotics are traditionally divided into high-, medium-, and low-potency drugs that are blocking the D2 and D1 dopamine receptors, with secondary blocking of the serotonine – histaminergic- adrenergic – and cholinergic receptors. The main drugs in this category are fluopentixole, haloperidole, zuclopenthizole, chlorpromazine and levopromazine. Side effects are unavoidable as the drugs block a range of receptor types. The side effects are extrapyramidal (dopaminergic – acute dystonia, parkinsonism, akathasia and tardive dyskinesia), serotonine related (weight gain), histaminergic (sedation), autonomic (hypotension, ejaculation failure, salivation), and anticholinergic (dry mouth, urine retention, constipation and blurred vision). A new class of antipsychotics which do not fit into the high/low potency classification have been introduced and are gaining world wide use because of their low degree of side effects. Drugs without cholinergic activity do not lead to extrapyramidal side effects. Some drugs have less adrenergic activity and they are generally more limbic than pyramidal in their selectivity compared to the classical antipsychotics. The main drugs in this category are: Amisulpride, risperidone, sertindole, closapine and olanzapine. Nervous and stress-related personality disorders (Neuroses) Phobic anxiety neurosis Anxiety neurosis is a chronic condition or it occurs as attacks of panic. Acute overactivity of the sympathoadrenergic system results in precardial pain and palpitations (cardiac neurosis = neurocirculatory asthenia), chest constriction, flatulence and frequent defecation and urination, lack of libido, dizziness, headache, and sleep disturbances. Attacks of panic anxiety occur in young, nervously sweating persons, who feel that they are dying from cardiac disease or from hyperventilation with tetany and carpopedal spasms. Hyperventilation reduces the carbon dioxide tension in the alveolar air (decreased PACO2) and thus the Ca2+ -concentration in the ECV, which opens Na+-channels, reduces the membrane potential and increases the neuromuscular irritability (see tetany in Chapter 17). Symptoms and signs of anxiety (ie, sweating, palpitations, tremor, tachycardia, flatulence, and urination) are caused by increased release of adrenaline and noradrenaline from the adrenal medulla. Drugs containing b -adrenergic blockers are of benefit to the anxious patient, because they block the sympathetic nerves and adrenergic synapses in the CNS. Cognitive-behavioural therapy is also applied with effect – sometimes combined with selective serotonin reuptake inhibitors (SSRI). Actually, SSRI substances are the first choice in anxiety conditions. Obsessive-compulsive disorders These patients have obsessional thoughts and perform compulsive actions to the extent that their social lives are seriously impaired. The patient feels an irresistible obsession to perform a given act - such as washing the hands or superstitious check of a closed door - again and again. To the patient the behaviour is often quite meaningless, but still it is necessary to carry on the ritual. A frequent complaint is that dirt and excretions are nasty, and many obsessions concern excretory processes. In cognitive-behaviour therapy the patients learn not to perform the compulsive rituals. Anorexia nervosa is an eating disorder in adolescent females resulting in severe malnutrition. The patient has an intense wish to be thin. Biological and psychological factors are involved. In a few cases there is regression into childhood. The girl or boy tries to escape from the problems of puberty and adolescence.The manifistation in females is amenorrhea and in males lack of libido and impotence The patients avoids fats and use self-induced vomiting plus execessive physical activity to reduce bodyweight. The body mass index (BMI) is less than 17.5. BMI is the weight of the person in kg divided by the height (in m) squared. The normal range is 19-25 kg per square metre. The increased concordance in monozygotic twins indicates a genetic aetiology. This disorder occurs among amenorrhoic teen-age girls, who express an abnormal fear of being fat. The girl is usually bright and knowledgeable. The patient sometimes realises the presence of problems in accepting the role as a maturing female and may have a distorted body view. The disorder involves the hypothalamo-hypophysary-gonadal axis. Low plasma Gonadotropin levels with impaired response to LHRH are frequently found. The patient may have high levels of growth hormone, cortisole and an abnormal insulin secretion. Positive reinforcement for even small weight gains is sometimes of help. The basic psychological problems must be treated with cognitive-behavioural or other psychological treatment. Tricyclic antidepressants are beneficial in cases of depression. Bulimia nervosa is diagnosed in persons who are preoccupied with food and periodically eats excessively. They may avoid overweight by self-induced vomiting just after binge eating, excessive physical activity and use of diuretics. Bulimia nervosa may be associated with anorexia nervosa. Behavioural therapy is sometimes successful – sometimes combined with selective serotonin reuptake inhibitors (SSRI). Both disorders react positive to ambulant therapy such as group therapy, which may allow the patient to live an almost normal life Dissociative-conversion disorders (hysteria) is characterised by psychologically mediated psycho-somatic disorders. These disorders have no physical pathology; they are not sympathetic overactivity and are produced without the consciousness of the patient. Freud believed that mental energy was converted into physical disorders such as abdominal pain, blindness, double vision, deafness, muteness, fits with dramatic movements, artistic gait disturbances, hysterical paresis with normal muscle tone and deep reflexes, crude tremor, sensory loss, stigmatisation, - all with secondary gain. The disorders are explained as the result of repression, dissociation and conversion of mental energy into physical disorders. Repression means exclusion of memories, impulses, and emotions from consciousness, because these elements would cause anxiety and distress. Dissociation means an apparent dissociation between different mental activities. An example is a protective mental cover of enjoyment in terminal cases of painful cancer (French: Belle indifference). The classical hysterical triad include mydriasis (large pupils), lack of pharyngeal reflexes and lack of plantar reflex. The mental disorders are amnesia for long periods, sleep walking or somnambulism (see below), imitation with multiple personalities, globus hystericus and pseudo-dementia. Psychotherapy is a causal treatment, although sometimes impossible to carry through. include insomnia, somnambulism and sleep apnoea. Insomnia is subjective sleep deficiency. The patient complains that he sleeps too little, or has the impression that he cannot sleep. Such patients sleep more than they think, when studied in sleep laboratories, and their health is not impaired. There is a natural decline of the sleep duration with age, and the use of drugs should be restrained. Monotonous sounds such as music or the sounds from ocean waves have proven to be an optimal "sleeping drug" for many individuals. The common complain of insomnia among the elderly is often curable by regular motion passes (eg, walking, swimming, jogging etc). Insomnia as early morning waking is a sign of depression, but it is also seen as nocturnal confusion in dementia and in delirium, where the cause may be organic brain damage or drug abuse. Sleepwalking or somnambulism is a form of personality dissociation with unknown aetiology. Some of the patients have hysterical patterns (see above). Sleep apnoea often occurs with snoring and airway obstruction in obese patients or in patients with chronic obstructive lung disease. Affect and stress reactions to psychological or physical stress are seen in otherwise healthy individuals. One example is described above as panic attacks. I. Each of the following five statements have True/False options: A. Cellular responses, mediated by drug receptors linked to ion-channels, are rapid compared to responses mediated by G-protein systems. B. Drugs acting via receptors have side effects, because they are bound to several receptors, distributed in several tissues, and the receptors are linked to different secondmessengers, which produce different cellular responses. C. Akathisia is an extrapyramidal defect with swaying and twisting body dyskinesia. D. The circumventricular organs (ie, hypothalamus, the pineal gland, and the area postrema have a tight blood-brain-barrier. E.. Dopamine agonists. such as amphetamine and other psychotic drugs (LSD, mescaline, ecstasy), can cause schizophrenic psychosis. II. Each of the following five statements have True/False options:
A professor in linguistics, 59 years old, consults his doctor because of speech and movement problems. The patient is intellectually well functioning, but his speech has changed from motivating to a slow monotonous sequence of words. His gait is slow with small steps, and the standing position is difficult for this previous long distance runner. His facial expression is motionless, and he seems to have difficulties in initiating normal movements. There is tremor of the hands and fingers of the pill-rolling type. When the doctor examines the patient for rigidity, he finds high tonus (plastic rigidity) and cogwheel-movements.
A female, 26 years of age, suffers from an epileptic seizure during her work as a nurse on a neurological department. A colleague saw that the nurse suddenly stopped while walking, her eyes and head turned left, her left hand moved in a curious way, and she uttered a cry and felled. The whole body became rigid for a minute, during which time she developed cyanosis. Then the muscles started to jerk rhythmically for a few minutes. She was unconscious during the seizure and remained so for an hour after the seizure. An EEG was taken during and after the seizure. A blood sample was taken and the blood glucose was determined to 5 mM.
A professor in economics, 56 years of age, finds it increasingly difficult to concentrate during his work. His wife and two adult children find him totally different from his normal personality; he is with- drawn, depressed and forgetful. Two months on antidepressants prescribed by his GP does not improve the condition, which is dominated by lack of memory. Psychiatric and neurological examination disclose no evidence for depression or increased intracranial pressure due to focal brain damage. CT scan shows cortical atrophy and excludes brain tumours. The mental powers are rapidly deteriorating. The total cholesterol concentration in blood plasma is increased.
Try to solve the problems before looking up the answers.
The Journal of Neuroscience. Semi-monthly journal published by the Society for Neuroscience, 11 Dupont Circle, NW, Washington DC 20036, USA. Hopkins AP (1993) Clinical Neurology, a Modern Approach. Oxford University Press, Oxford. Sims ACP and DW Owens (1993) Psychiatry. 6th edition. London: Bailliere Tindall.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Click here to introduce your comments or contributions